silver coulometer → srebrni kulometar
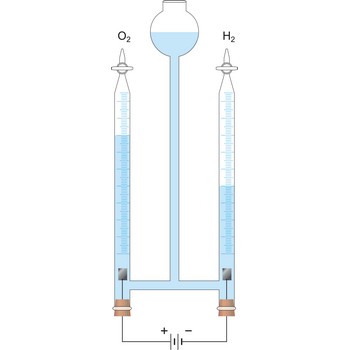
Silver coulometer consists of a platinum vessel which acts as a cathode and contains a solution of pure silver nitrate as an electrolyte (c(AgNO3) = 1 mol/L). A rod of pure silver enclosed in a porous pot acts as the anode. The current density at the anode should not exceed 0.2 Acm-2. After electrolysis, the electrolyte is taken out and the platinum vessel is washed, dried and weighed. The increase in the weight gives the amount of silver deposited (96500 C of electricity deposits 107.88 g of silver). From the mass of the silver deposited, the coulomb involved in the reaction can be calculated.
styrene → stiren
Styrene is an unsaturated hydrocarbon (C6H5OC2H3O) colourless, toxic liquid with a strong aromatic aroma. It is soluble in alcohol, ether, acetone, and carbon disulfide, but dissolves only slightly in water. It is used to make plastics such as polystyrene, ABS, styrene-butadiene rubber styrene-butadiene latex and unsaturated polyesters.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
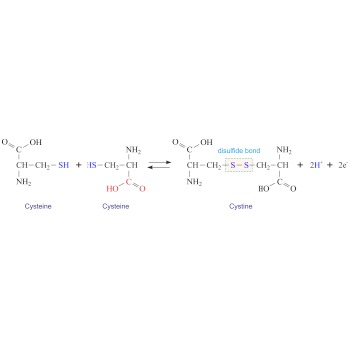
Cystine → Cistin
Cystine (C6H12N2O4S2) is a dimer of cysteine. It is formed by the oxidation of the thiol groups (-SH) of two cysteines generating a disulphide bridge (-S-S-). Cystine is a white crystalline solid that is slightly soluble in water. Cystine is particularly abundant in skeletal and connective tissues and in hair, horn, and wool.
Citing this page:
Generalic, Eni. "Slabi elektrolit." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table