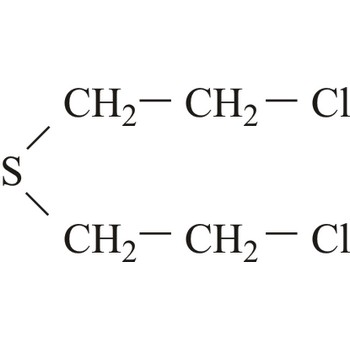lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
mass-energy equivalence → ekvivalencija mase i energije
In the special theory of relativity Einstein demonstrated that neither mass nor energy were conserved separately, but that they could be traded one for the other and only the total "mass-energy" was conserved. The relationship between the mass and the energy is contained in what is probably the most famous equation in science,
Where m is the mass of the object and c is the velocity of light. Cockcroft and Walton (1932) are routinely credited with the first experimental verification of mass-energy equivalence.
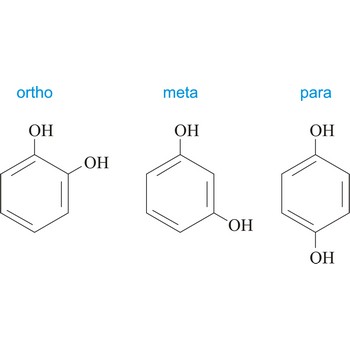
meta position → meta položaj
Meta position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in position 1 and 3. The abbreviation m- is used, for example, m-Hydroquinone is 1,3-dihydroxybenzene.
methionine → metionin
Methionine is neutral amino acids with polar side chains. It is one of the two sulfur-containing amino acids. Methionine is a fairly hydrophobic amino acid and typically found buried within the interior of a protein. It can form stacking interactions with the aromatic moieties of tryptophan, phenylalanine, and tyrosine. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Met, M
- IUPAC name: 2-amino-4-methylsulfanylbutanoic acid
- Molecular formula: C5H11NO2S
- Molecular weight: 149.21 g/mol
microscope → mikroskop
Microscope is an instrument that produces enlarged images of small objects. The optical microscopes (light microscope) use visible light and a system of lenses to magnify images. Typical magnification of a light microscope is up to 1500× ("1500 times")with a theoretical resolution limit of around 200 nm. Instead of using light, electron microscopes transmit a beam of electrons through, or onto the surface of, a specimen. An electron beam has a much shorter wavelength than does light, and can reveal structures as small as 2 nm.
mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
Citing this page:
Generalic, Eni. "Skupine periodnog sustava." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table