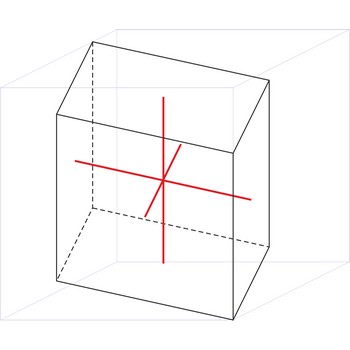
orthorhombic crystal system → ortorompski kristalni sustav
Orthorhombic crystal system is also known as the rhombic system. Minerals of the orthorhombic crystal system are referred to three mutually perpendicular axes, each of which is of a different length than the others.
a ≠ b ≠ c
α = β = γ = 90°
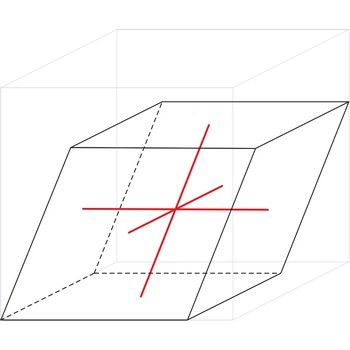
rhombohedral crystal system → romboedarski kristalni sustav
Rhombohedral crystal system is also known as the trigonal system. The crystallographic axes used in this system are of equal length. None of the axes are perpendicular to any other axis.
a = b = c
α= β = γ ≠ 90°
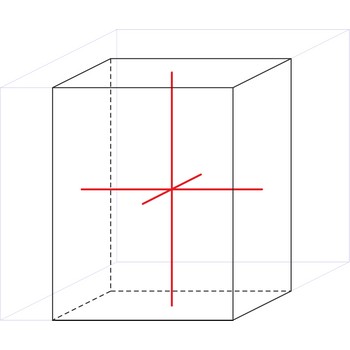
tetragonal crystal system → tetragonski kristalni sustav
Minerals of the tetragonal crystal system are referred to three mutually perpendicular axes. The two horizontal axes are of equal length, while the vertical axis is of different length and may be either shorter or longer than the other two.
a = b ≠ c
α = β = γ = 90°
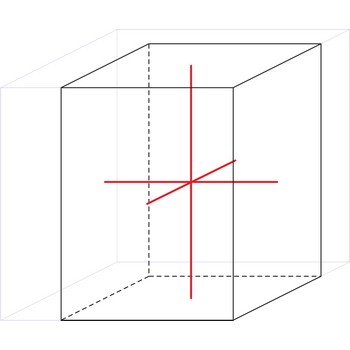
triclinic crystal system → triklinski kristalni sustav
Minerals of the triclinic crystal system are referred to three unequal axes, all of which intersect at oblique angles. None of the axes are perpendicular to any other axis.
a ≠ b ≠ c
α ≠ β ≠ γ ≠ 90°
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
allomerism → alomerija
Allomerism is the appearance of substances with different chemical composition but the same crystalline form.
allomorphism → alomorfija
Allomorphism is the existence of chemical substances with same chemical composition in two or more crystalline forms. See Polymorphism.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
Citing this page:
Generalic, Eni. "Savršeni kristal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table