chlorofluorocarbons → klorofluorougljici
Chlorofluorocarbons (CFC) are manmade chemicals containing chlorine, fluorine, and carbon. CFCs are used for industrial purposes and in the home for refrigeration, air conditioning, aerosols and as a raw material in polystyrene production.
colligative properties → koligativna svojstva
Colligative properties are properties which affect a solvent based on the number of molecules of solute present such as melting point, boiling point and osmotic pressure.
colloid silver → koloidno srebro
Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour.
colorimeter → kolorimetar
Colorimeter is an instrument used to measure the strength of colorification in a solution.
colorimetry → kolorimetrija
Colorimetry is a quantitative chemical analysis by colour using a colorimeter.
beta-glucan → beta-glukan
Beta-glucans are are naturally occurring polysaccharides that contain only glucose as structural components, and are linked with β-glycosidic bonds. They is the most known powerful immune stimulant. The most active forms of β-glucans are those comprising D-glucose units with β(1→3) links and with short side-chains of D-glucose attached at the β(1→6) position. These are referred to as beta-1,3/1,6 glucan. They are a major component of soluble dietary fiber, which can be found in cereal grains (oats, barley, wheat), yeast, and certain mushrooms (shiitake, maitake).
bromine → brom
Bromine was discovered by Antoine J. Balard (France) in 1826. The origin of the name comes from the Greek word bromos meaning stench. It is reddish-brown liquid with suffocating, irritating fumes. Gives off poisonous vapour. Causes severe burns. Oxidizer. Bromine occurs in compounds in sea water. It was once used in large quantities to make a compound that removed lead compound build up in engines burning leaded gasoline. Now it is primarily used in dyes, disinfectants and photographic chemicals.
Bunsen’s cell → Bunsenov članak
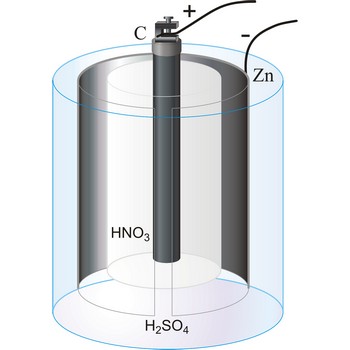
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
burette → bireta
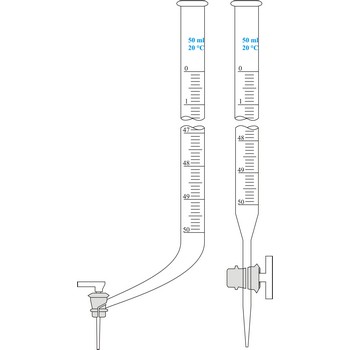
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
Citing this page:
Generalic, Eni. "Sastav otopine." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table