diagenesis → dijageneza
Diagenesis is the process that turns sediments into sedimentary rocks. The lithification (literally turning into stone) of the sediments is usually accomplished by a cementing agent. How the weight of the overlying material increases the grains closer together, reducing pore space and eliminating some of the contained water. This water may carry mineral components in solution, and these constituents precipitate as new minerals in the pore spaces. This causes cementation, which will then start to bind the individual particles together. Further compaction and burial may cause recrystallization of the minerals to make the rock even harder.
diatomaceous earth → dijatomejska zemlja
Diatomaceous earth is a naturally occurring siliceous sedimentary mineral compound from microscopic skeletal remains (frustules) of diatoms, unicellular aquatic plants of microscopic size. Their fossilized remains are called diatomite and contains approximately 3000 diatom frustules per cubic millimetre.
Diatomite is relatively inert and has a high absorptive capacity, large surface area, and low bulk density. It consists of approximately 90 % silica, and the remainder consists of compounds such as aluminum and iron oxides. The fine pores in the diatom frustules make diatomite an excellent filtering material for waters, beverages, oils, chemicals, as well as many other products.
pigment → pigment
Pigments are the substances that give paint colour. Pigments are derived from natural or synthetic materials that have been ground into fine powders. A pigment is different from a dye in that a pigment is insoluble in the media in which it is used.
Pigment is an organic substance found in plant and animal cells that creates colouring.
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
differential thermal analysis → diferencijalna termalna analiza
Differential thermal analysis (DTA) is a technique that is often used to analyze materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample.
electrodeposition → elektrodepozicija
Electrodeposition is a process of depositing solid materials on an electrode surface using electrolysis. It is a somewhat loosely used term that is applied to many technologies. There are a number of metal deposition technologies. However, not only metals but also different compounds can be electrodeposited. This is used most often for the formation of oxides (such as manganese dioxide and lead dioxide) by anodic oxidation of dissolved salts.
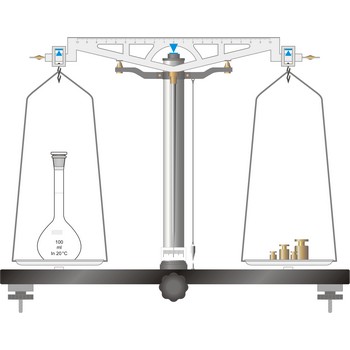
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
purification → pročišćavanje
Purification is the physical or chemical process of removing contaminants from a compound. The physical processes may include sublimation, distillation, filtration, crystallisation, or extraction. The chemical processes may involve formation of a derivative, purification of the derivative and recovery of the original material in a pure form of the derivative.
ferrite → ferit
Ferrites are ceramic materials of the nominal formula MO·Fe2O3, where M is a divalent metal (Co, Mn, NI, or Zn). The ferrites show either ferrimagnetism or ferromagnetism, but are not electrical conductors, and they are used in high-frequency circuits as magnetic cores, in rectifiers on memory and record tapes, and various related uses in radio, television, radar, computers, and automatic control systems.
Citing this page:
Generalic, Eni. "Samozapaljivi materijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table