dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
electrolysis → elektroliza
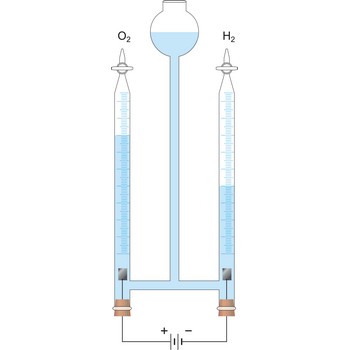
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
electrolytic cell → elektrolitska ćelija
Electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. The chemical reactions do not occur spontaneously at the electrodes when they are connected through an external circuit. The reaction must be forced by applying an external electric current. It is used to store electrical energy in chemical form (rechargeable battery). It is also used to decompose or produce (synthesise) new chemicals by the application of electrical power. This process is called electrolysis, e.g., water can be decomposed into hydrogen gas and oxygen gas. The free energy change of the overall cell reaction is positive.
precipitate → precipitat
Precipitate or the deposit is an insoluble solid formed by reactions in a solution. For example, when a solution of silver nitrate is added to a solution of sodium chloride, insoluble silver chloride precipitates.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
electron affinity → elektronski afinitet
Electron affinity (EA) is the energy change occurring when an atom or molecule gains an electron to form a negative ion. For an atom or molecule X, it is the energy released for the electron-attachment reaction
This is often measured in electronvolts. Alternatively, the molar enthalpy change, ΔH, can be used.
electroplating → galvaniziranje
Electroplating (also called electrodeposition) is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to reduce the positively charged ions to metallic form.
Typically, a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode. The anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is a mixure of silver nitrate with potassium cyanide. The reactions are:
The cyanide ensures a low concentration of silver ions, a condition for providing the best plating results.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
Citing this page:
Generalic, Eni. "Reverzibilna reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table