poison → otrov
Poisons are substance, which upon contact or being introduced into an organism, impair or prevent normal metabolic processes from taking place, thus altering the normal functioning of organs or tissues.
Poisons are molecules or material that tends to collect on a catalyst surface, blocking access to active sites or destroying their activities.
Poisons are substance that can reduce a nuclear reaction by absorbing neutrons, thereby preventing more fission. If enough poisons are present in a reactor core, the chain reaction will die out.
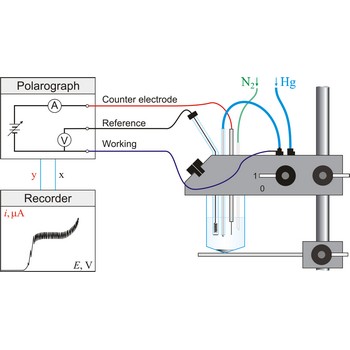
polarography → polarografija
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
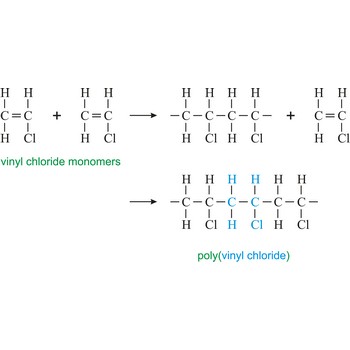
polymerization → polimerizacija
Polymerization is a reaction of connecting many monomers in one long molecule whereby polymers are created.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
radioactive indicator → radioaktivni indikator
By use of suitable radioactive isotopes biochemical processes can be observed in plants, animals and humans, by measuring radioactive radiation of radioactive indicator. Artificial radioactive isotopes have the same chemical properties as natural ones, which enable us to mark those natural isotopes with addition of artificial ones and in this way follow the path of those elements during a chemical reaction. One of the most important radioactive indicators is the radioactive carbon 14C.
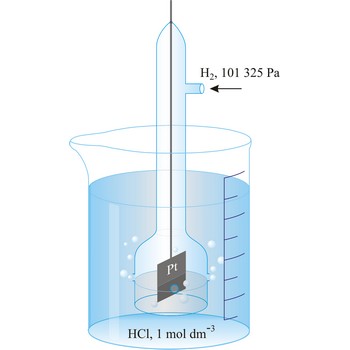
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
redox titration → redoks-titracija
Redox titration (oxidation-reduction titration) is a titration based on a redox reaction. For example, iron in water can be determined by converting dissolved iron to Fe2+ and titrating the solution with potassium permanganate (KMnO4), a powerful oxidising agent.
reducing agent → redukcijsko sredstvo
Reducing agent may be defined in various ways, depending upon the context in which the phrase is used. In broad terms it is often taken to mean a chemical which can act as an electron donor. Thus, in the reaction:
the zinc is being reduced (gaining electrons) by reaction with the iron cations; the Fe2+ in this instance is acting as a reducing agent.
scandium → skandij
Scandium was discovered by Lars Fredrik Nilson (Sweden) in 1879. The origin of the name comes from the Latin word Scandia meaning Scandinavia. It is fairly soft, silvery-white metal. Burns easily. Tarnishes readily in air. Reaction with water releases hydrogen. Reacts with air and halogens. Scandium occurs mainly in the minerals thortveitile (~34 % scandium) and wiikite. Also in some tin and tungsten ores. Pure scandium is obtained as a by-product of uranium refining. Scandium metal is used in some aerospace applications. Scandium oxide (Sc2O2) is used in the manufacture of high-intensity electric lamps. Scandium iodide (ScI3) is used in lamps that produce light having a colour closely matching natural sunlight.
silicon → silicij
Silicon was discovered by Jöns Jacob Berzelius (Sweden) in 1824. The origin of the name comes from the Latin word silicis meaning flint. Amorphous form of silicon is brown powder; crystalline form has grey metallic appearance. Solid form unreactive with oxygen, water and most acids. Dissolves in hot alkali. Silica dust is a moderately toxic acute irritant. Silicon makes up major portion of clay, granite, quartz and sand. Commercial production depends on a reaction between sand (SiO2) and carbon at a temperature of around 2200 °C. Used in glass as silicon dioxide (SiO2). Silicon carbide (SiC) is one of the hardest substances known and used in polishing. Also the crystalline form is used in semiconductors.
Citing this page:
Generalic, Eni. "Reverse reaction." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table