fouling → obrastanje
1. Fouling is the deposition of insoluble materials, such as bacteria, colloids, oxides and water-borne debris, onto the surface of a reverse osmosis or ultrafiltration membrane. Fouling is associated with decreased flux rates and may also reduce the rejection rates of reverse osmosis membranes. Fouling also refers to the accumulation of normally inorganic deposits on heat exchanger tubing.
2. Fouling is an accumulation of marine organism deposits on a submerged metal surface.
colloid ion → koloidni ion
Colloid ions emerge when colloid particles adsorb certain type of ion from solution and thus become charged with the same charge. The charge can also originate form a chemical reaction of colloid particle’s surface. Colloid ions formed by absorption of silver chloride particle can be show as follows:
Adsorbed layer is monomolecular (one molecule thick) and which type of ion will be formed depends upon which ions are present in a greater number in the solution in. Because of this colloid particles are charged with the same charge, mutual repelling occurs, and the colloid solution becomes stable. Colloid charge can be determined by electrophoresis.
condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
glass transition temperature → temperatura staklastog prijelaza
Glass transition temperature (Tg) is the temperature at which an amorphous polymer is transformed, in a reversible way, from a viscous or rubbery condition to a hard and relatively brittle one.
Helmholz free energy → Helmholzova slobodna energija
Helmholz free energy (A) is a thermodynamic function defined by A = U - TS, where U is the internal energy, S the entropy, and T the thermodynamic temperature. For a reversible isothermal process ΔA represents the useful work available.
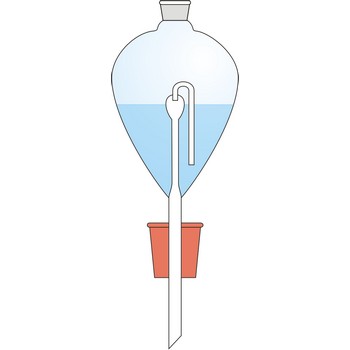
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
darmstadtium → darmstadtij
Darmstadtium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in November 1994. The title honours the Laboratory for Heavy Ion Research (called GSI) in Darmstadt, Germany, where the substance was first made. It is synthetic radioactive metal. The fusion-evaporation reaction using a 62Ni beam on an isotopically enriched 208Pb target produced four chains of alpha-emitting nuclides following the presumed formation of 269110 + 1n.
Citing this page:
Generalic, Eni. "Reverse reaction." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table