inert electrode → inertna elektroda
Inert electrode is an electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction. Precious metals, mercury, and carbon are typically used as inert electrodes. The inert nature of the electrode can sometimes be questioned. While the electrode may not take part in the reaction as a reactant or product, it still can act as an electrocatalyst.
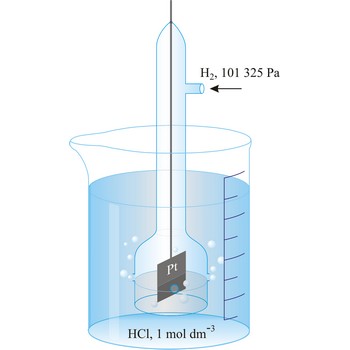
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
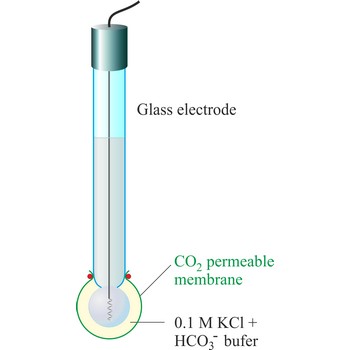
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
electrodeposition → elektrodepozicija
Electrodeposition is a process of depositing solid materials on an electrode surface using electrolysis. It is a somewhat loosely used term that is applied to many technologies. There are a number of metal deposition technologies. However, not only metals but also different compounds can be electrodeposited. This is used most often for the formation of oxides (such as manganese dioxide and lead dioxide) by anodic oxidation of dissolved salts.
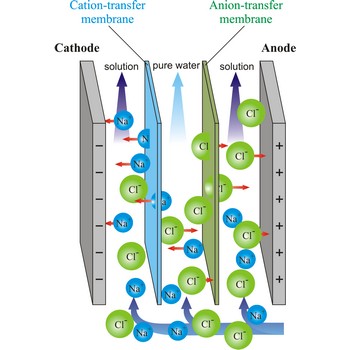
electrodialysis → elektrodijaliza
Electrodialysis is a procedure of dialysis accelerated with an electric field. Dialyser is divided into three sections. Solution flows through the middle section, between two semipermeable membranes alternately to positive ions and negative ions. An electrodes are placed in the neighbouring sections. Under the influence of electric field, positive ions will travel towards the cathode (the negative electrode), and negative ions towards the anode (the positive electrode), whereby travelling of ions through the membrane is accelerated. In this way, the feed water is separated into two streams: one of pure water and the other of more concentrated solution.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
inertial reference frames → inercijski referentni sustavi
When two frames of reference are moving relative to each other at constant velocity, they are said to be inertial reference frames. The observers from two such inertial frames measure, in general, different velocities of a moving particle. On the other hand, they measure the same acceleration for the particle. The laws of physics must have the same form in all inertial reference frames (the principle of invariance).
ion selective electrode → ion selektivne elektrode
Ion selective electrode (ISE) is an electrode or electrode assembly with a potential that is dependent on the concentration of an ionic species in the test solution and is used for electroanalysis. Ion-selective electrodes are often membrane type electrodes.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
Citing this page:
Generalic, Eni. "Referentna elektroda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table