neutralisation → neutralizacija
Neutralisation is the process in which an acid reacts with a base to form a salt and water.
oxidating agent → oksidans
Oxidating agent is a substance that receives electrons and oxidates other substances. Oxidating agent is always reduced in this reaction.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
polycondensational polymer → polikondenzacijski polimer
Polycondensational polymers are compounds which are obtained by condensation polymerization with successive repetitions of the condensation reaction.
precipitate → precipitat
Precipitate or the deposit is an insoluble solid formed by reactions in a solution. For example, when a solution of silver nitrate is added to a solution of sodium chloride, insoluble silver chloride precipitates.
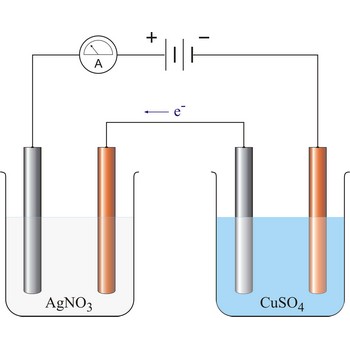
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
racemisation → racemizacija
Racemisation is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its miror image (enantiomer).
Citing this page:
Generalic, Eni. "Redoks-reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table