Haber process → Haberov proces
Haber process is an industrial process for producing ammonia by reaction of nitrogen with hydrogen:
The reaction is reversible and exothermic, so that a high yield of ammonia is favoured by low temperature. However, the rate of reaction would be too slow for equilibrium to be reached at normal temperatures, so an optimum temperature of about 450 °C is used, with a catalyst of iron containing potassium aluminium oxide promoters. The higher the pressure the greater the yield, although there are technical difficulties in using very high pressures. A pressure of about 250 atmospheres is commonly employed. The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained. The nitrogen is obtained from air. Formerly, the hydrogen was from water gas and the water-gas shift reaction (the Bosch process) but now the raw material (called synthesis gas) is obtained by steam reforming natural gas.
The process is of immense importance for the fixation of nitrogen for fertilisers and explosives. It was developed in 1908 by German chemist Fritz Haber (1868-1934) and was developed for industrial use by Carl Bosch (1874-1940), hence the alternative name Haber-Bosch process.
holmium → holmij
Holmium was discovered by Per Theodore Cleve (Sweden) in 1879. The origin of the name comes from the Greek word Holmia meaning Stockholm. It is fairly soft, malleable, lustrous, silvery metal. Reacts slowly with oxygen and water. Dissolves in acids. Can react violently with air or halogens. Holmium occurs in gadolinite. Most often from monazite sand. It has very few practical applications; however, it has some unusual magnetic properties that offer some hope for future applications.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
invert sugar → invertni šećer
Invert sugar is a mixture of equal parts of glucose and fructose resulting from the hydrolysis of sucrose (saccharose). The name stemming from the fact that it rotates of plane polarized light in the opposite direction of sucrose. Sucrose is dextrorotatory - it rotates polarized light clockwise ([α]D = +66.5°). Invert sugar rotates the plane of the polarized light counterclockwise ([α]D = -22°) due to the strongly levorotatory nature of fructose ([α]D = -92°).
Homemade artificial honey (invert sugar syrup): Dissolve two parts of household sugar (1 kg) with stirring in one part of water (0.5 kg) in a saucepan over low heat. Add 1 g of citric acid or the juice of one lemon to the mixture. Bring the ingredients to a slow boil. It can take anywhere between 15 minutes to 1 hour. The end result is sticky, golden syrup. Let it sit at room temperature until it is cool.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
neodymium → neodimij
Neodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words neos didymos meaning new twin. It is silvery-white, rare-earth metal that oxidizes easily in air. Reacts slowly in cold water, more rapidly as heated. Metal ignites and burns readily. Neodymium is made from electrolysis of its halide salts, which are made from monazite sand. Used in making artificial ruby for lasers. Also in ceramics and for a special lens with praseodymium. Also to produce bright purple glass and special glass that filters infrared radiation. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 18 % neodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4). Neodymium is used to create some of the most powerful permanent magnets on Earth, known as NIB magnets they consist of neodymium, iron, and boron.
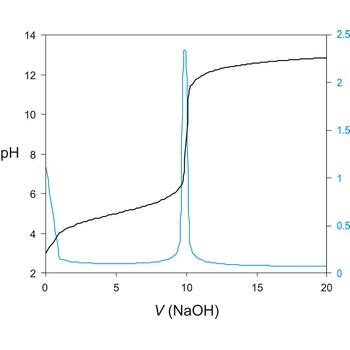
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
Tafel plot → Tafelov dijagram
Tafel plot is the graph of the logarithm of the current density j against the overpotential η in electrochemistry in the high overpotential limit. An electrode when polarised frequently yields a current potential relationship over a region which can be approximated by:
where η is change in open circuit potential, i is the current density, B and i0 is constants. B is known as the Tafel Slope. If this behaviour is observed a plot of the semilogarithmic components is known as the Tafel line and the diagram is called the Tafel diagram.
Citing this page:
Generalic, Eni. "Reakcijski sloj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table