zero-order reaction → reakcija nultog reda
Zero-order reaction is a reaction for which the rate of reaction is independent of the concentration of reactants.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
Acheson process → Achesonov proces
Acheson process is an industrial process to synthesize graphite and silicon carbide (carborundum), named after its inventor the American chemist Edward Goodrich Acheson (1856-1931). In this process, a solid-state reaction between pure silica sand (SiO2) and petroleum coke (C) at very high temperature (more than 2500 °C) leads to the formation of silicon carbide under the general reaction:
While studying the effects of high temperature on carborundum, Acheson had found that silicon vaporizes at about 4150 °C, leaving behind graphitic carbon.
acid dissociation constant → konstanta disocijacije kiseline
Acid dissociation constant (Ka) is the equilibrium constant for the dissociation of an acid HA through the reaction
The quantity pKa = -log Ka is often used to express the acid dissociation constant.
acid rain → kisela kiša
Acid rain is rainwater that shows acid reaction because of nitrogen and sulphur oxides absorption. It is generated mainly by industrial pollutions.
activated complex → aktivirani kompleks
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
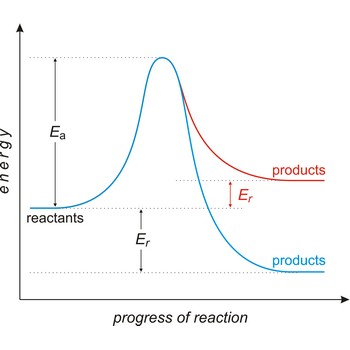
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
Citing this page:
Generalic, Eni. "Reakcije aciliranja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table