supersaturated solution → prezasićena otopina
Solution is supersaturated when it contains greater quantity of dissolved substance in itself than it corresponds to solubility of that substance at that temperature. It is said to be in an unstable state, and by shaking the vessel containing that such a solution separation of salt surplus can occur.
unsaturated solution → nezasićena otopina
Unsaturated solution is a solution that contains less than the maximum possible equilibrium concentration of a solute.
ideal solution → idealna otopina
Ideal solution is a solution in which solvent-solvent and solvent-solute interactions are identical, so that properties such as volume and enthalpy are exactly additive. Ideal solutions follow Raoult’s law, which states that the vapour pressure pi of component i is pi = xi pi*, where xi is the mole fraction of component i and pi* the vapour pressure of the pure substance i.
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
buffer capacity → puferski kapacitet
Buffer capacity is number of moles of a strong acid or a strong base needed to change pH of 1 dm3 of buffer solution for pH unit.
solid solution → čvrste otopine
Solid solution is a crystalline material that is a mixture of two or more components, with ions, atoms, or molecules of one component replacing some of the ions, atoms of the other component in its normal crystal lattice.
ionic strength → ionska jakost otopine
Ionic strength (μ or I) is a measure of the total concentration of ions in a solution, defined by
where zi is the charge of ionic species i and ci is its concentration.
solution composition → sastav otopine
Solutions are homogenous mixtures of several components. The component which is found in a greater quantity is called the solvent and the other components are called solutes. Quantitative composition of a solution can be expressed by concentration (amount, mass, volume and number), by fraction (amount, mass, and volume), ratio (amount, mass, and volume) and by molality. Amount, mass, and volume ratio are numerical, nondimensional units and are frequently expressed as percentage (% = 1/100), promile (‰ = 1/1000) or parts per million (ppm = 1/1 000 000). If it is not defined, it is always related to the mass ratio.
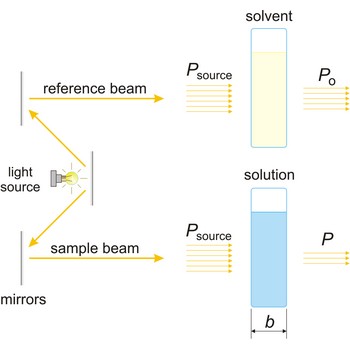
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Pufer otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table