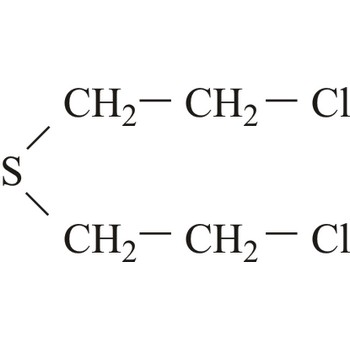ideal solution → idealna otopina
Ideal solution is a solution in which solvent-solvent and solvent-solute interactions are identical, so that properties such as volume and enthalpy are exactly additive. Ideal solutions follow Raoult’s law, which states that the vapour pressure pi of component i is pi = xi pi*, where xi is the mole fraction of component i and pi* the vapour pressure of the pure substance i.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
inertial reference frames → inercijski referentni sustavi
When two frames of reference are moving relative to each other at constant velocity, they are said to be inertial reference frames. The observers from two such inertial frames measure, in general, different velocities of a moving particle. On the other hand, they measure the same acceleration for the particle. The laws of physics must have the same form in all inertial reference frames (the principle of invariance).
Knudsen’s pipette → Knudsenova pipeta
Kudsen's automatic pipette, developed by the Danish physicist Martin Knudsen (1871-1949), allows quick and accurate transfer of a constant volume of liquid (sea water), usually around 15 mL. On the top of pipette is a double sided C vent that can establish flow between the body of the pipette and one of the branches (A or B), or isolate the body of the pipette from both of the branches. Sucking through the B branch the pipette is filled with liquid, it is closed with a twist of the C valve and the liquid is released by rotating the valve towards the A branch (so atmospheric air can enter the pipette). Emptying the pipette takes around 30 seconds. Before it's first use, the pipette must be calibrated with distilled water.
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
living planet index → indeks života na planetu
The Living Planet Index (LPI) reflects changes in the health of the planet's ecosystems by tracking trends in nearly 8000 populations of vertebrate species. The LPI first calculates the annual rate of change for each species population in the dataset, then calculates the average change across all populations for each year from 1970, when data collection began, to 2007, the latest date for which data is available.
The Global LPI shows a decline of around 30 % from 1970 to 2007, based on 7953 populations of 2544 species of birds, mammals, amphibians, reptiles and fish.
mass-energy equivalence → ekvivalencija mase i energije
In the special theory of relativity Einstein demonstrated that neither mass nor energy were conserved separately, but that they could be traded one for the other and only the total "mass-energy" was conserved. The relationship between the mass and the energy is contained in what is probably the most famous equation in science,
Where m is the mass of the object and c is the velocity of light. Cockcroft and Walton (1932) are routinely credited with the first experimental verification of mass-energy equivalence.
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
Citing this page:
Generalic, Eni. "Prvi zakon termodinamike." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table