spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
standard electrode potential → standardni elektrodni potencijal
Standard electrode potential (E°) (standard reduction potentials) are defined by measuring the potential relative to a standard hydrogen electrode using 1 mol solution at 25 °C. The convention is to designate the cell so that the oxidised form is written first. For example,
The e.m.f. of this cell is -0.76 V and the standard electrode potential of the Zn2+|Zn half cell is -0.76 V.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
technetium → tehnecij
Technetium was discovered by Carlo Perrier and Emilio Segre (Italy) in 1937. The origin of the name comes from the Greek word technikos meaning artificial. It is silvery-grey metal. Resists oxidation but tarnishes in moist air and burns in high oxygen environment. First synthetically produced element. Radioactive. Technetium is made first by bombarding molybdenum with deuterons (heavy hydrogen) in a cyclotron. Added to iron in quantities as low as 55 part-per-million transforms the iron into a corrosion-resistant alloy.
teflon → teflon
Teflon is a DuPont Company trademark for polytetrafluoroethylene (PTFE). It was discovered in 1938 by R. J. Plunkett. Teflon has the lowest coefficient of friction of any solid material known to man. It is also used as a non-stick coating for pans and other cookware. Teflon is very unreactive, and so is often used in containers and pipework for reactive chemicals. Its melting point is 327 °C.
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
thermal resistance → toplinski otpor
Heat always flows from a higher to a lower temperature level. The driving force for the heat flux lies in the temperature difference ΔT between two temperature levels. Analogous to Ohm’s law, the following holds:
where H = dQ/dt is heat flux, measured in watts, ΔT is temperature difference across the thermal resistance, measured in kelvin, and Rth is thermal resistance, measured in K/W.
For example, suppose there were two houses with walls of equal thickness; one is made of glass and the other of asbestos. On a cold day, heat would pass through the glass house much faster. The thermal restistance of asbestos is then higher than of glass.
If the thermal Ohm’s law is divided by the heat capacity C, Newton’s law of cooling is obtained:
where dT/dt is rate of cooling or heating, measured in K s-1, and C is heat capacity, measured in J K-1.
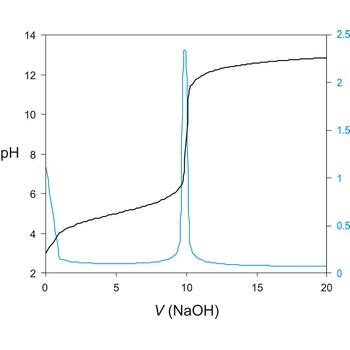
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
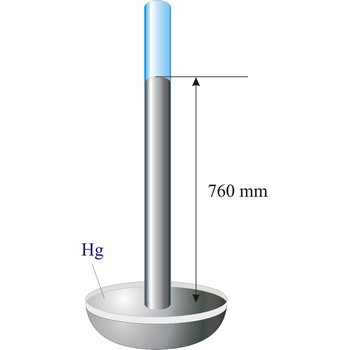
Torricelli, Evangelista → Torricelli, Evangelista
Evangelista Torricelli (1852-1908) is Italian physicist and mathematician. He became the first scientist to create a sustained vacuum and to discover the principle of a barometer. He filled a tube three feet long, and hermetically closed at one end, with mercury and set it vertically with the open end in a basin of mercury, taking care that no air-bubbles should get into the tube. The column of mercury invariably fell to about twenty-eight inches, leaving an empty space above its level. He discovered that the variation of the height of the mercury from day to day was caused by changes in the atmospheric pressure. He also constructed a number of large objectives and small, short focus, simple microscopes.
Citing this page:
Generalic, Eni. "Prvi zakon termodinamike." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table