mass concentration → masena koncentracija
Mass concentration (γ) is equal to mass (mA) of soluted substance and volume (V) of the solution proportion. SI unit for mass concentration is kg m-3, but in laboratory practice g dm-3, which has the same number value, is often used.
mass fraction → maseni udio
Mass fraction (wA) is the ratio of the mass of substance A to the total mass of a mixture.
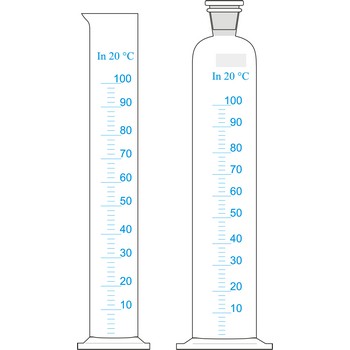
measuring cylinder → menzura
Measuring cylinders (graduated cylinders) are graduated glass cylinders with a capacity from 2 mL to 2 L. They are used when reagent solutions for volume measuring are prepared when accuracy is not of great relevance. The larger the measuring cylinder, the bigger the measuring error.
molybdenum → molibden
Molybdenum was discovered by Carl William Scheele (Sweden) in 1778. The origin of the name comes from the Greek word molybdos meaning lead. It is silvery white, very hard metal, but is softer and more ductile than tungsten. Molybdenum is found in the minerals molybdenite (MoS2) and wulfenite (MoO4Pb). Its alloys are used in aircraft, missiles and protective coatings in boiler plate.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
osmotic pressure → osmotski tlak
Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution
where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
pH → pH
pH is a convenient measure of the acid-base character of a solution, usually defined by
where c(H+) is the concentration of hydrogen ions in moles per litre. The more precise definition is in terms of activity rather than concentration.
A solution of pH 0 to 7 is acid, pH of 7 is neutral, pH over 7 to 14 is alkaline.
pickling → dekapiranje
Pickling is a process to chemically remove scale or oxide from steel to obtain a clean surface. When applied to bars or coils prior to bright drawing, the steel is immersed in a bath of dilute sulphuric acid (w(H2SO4) = 10 %) heated to a temperature of around 80 °C. An inhibitor is added to prevent attack and pitting of the cleaned metal. After pickling, a washing process takes place followed by immersion in a lime-water bath to neutralise any remaining acid.
Citing this page:
Generalic, Eni. "Prezasićena otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table