ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
substitution → supstitucija
Substitution (substitution reaction) is a reaction in which an atom or fragment within a molecule is replaced with another atom or a fragment.
substrate → supstrat
1. Substrate is a surface upon which an organism grows, sometimes by using chemicals of particles in the material as food
2. Substrate is a substance that is acted upon by an enzyme during a biochemical reaction.
3. Substrate is the material or product that is to be coated (for example, paint or laminate.).
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
Nernst’s electrode potential equation → Nernstova jednadžba za elektrodni potencijal
For general reaction of some redox system
dependence of electrode potential of redox system upon activity of oxidised and reduced form in solution is described in Nernst’s equation for electrode potential:
where E = to electrode potential of redox system
E° = standard electrode potential of redox system
R = universal gas constant
T = thermodymical temperature
F = Faraday’s constant
z = number of electrons exchanged in redox reaction
aO = activity of oxidised form
aR = activity of reduced form
n = stechiometrical coefficient of oxidised form
m = stechiometrical coefficient of reduced form
termination → terminacija
Termination is the final step in a free radical mechanism that results in the stopping of the free radical reaction.
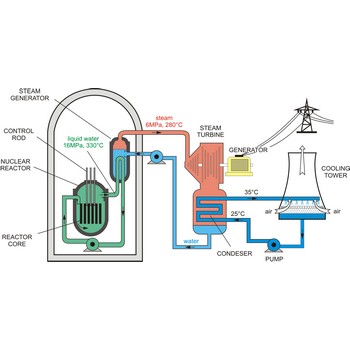
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
Citing this page:
Generalic, Eni. "Povratna reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table