kinetic theory → kinetička teorija
Kinetic theory explains the behaviour of solids, liquids and gases and their state changes dependable upon motion of particles they are made of.
latent heat → latentna toplina
Latent heat (L) is the quantity of heat absorbed or released when a substance changes its physical phase at constant temperature (e.g. from solid to liquid at the melting point or from liquid to gas at the boiling point).
millimetre of mercury → milimetar živina stupca
Millimetre of mercury (mmHg) is a non-SI unit of pressure, equal to 133.322 Pa. The name is generally considered interchangeable with torr.
mineral oils → mineralna ulja
Mineral oils are oily liquids that are composed of hydrocarbons and are obtained as a product of petroleum, tar, coal, wood etc. distillation. They are used as lubricants.
filter paper → filtar papir
Filter paper is a quantitative paper used for filtering and made of pure cellulose treated with hydrochloric and hydrofluoric acid. This kind of paper burns out practically without any remains (less than 0.0001 g ashes). Different types of paper are marked with numbers; qualitative bears marking 595 or 597 and quantitative 589 or 590. Dependable upon precipitate character, different types of filter paper are used:
- black band (5891) - 100 mL of fluid flows through it in 20 s to 30 s. It is used for filtering of gelatinous precipitates.
- white band (5892) - 100 mL of fluid flows through it in 40 s to 60 s. It is used for coarse crystalline precipitates filtration.
- blue band (5893) - 100 mL of fluid flows through it in 200 s to 400 s. It is used for fine crystalline precipitates.
filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
foam → pjena
Foams are dispersions of gases in liquids or solids. The gas globule may be of any size, from colloidal to macroscopic, as in soap bubbles. Bakers’ bread and sponge rubber are examples of solid foams. Typical liquid foams are those used in fire-fighting, shaving creams, etc. Foams made by mechanical incorporation of air are widely used in the food industry (e.g. whipped cream, egg white, ice cream, etc.). Foams can be stabilized by surfactants.
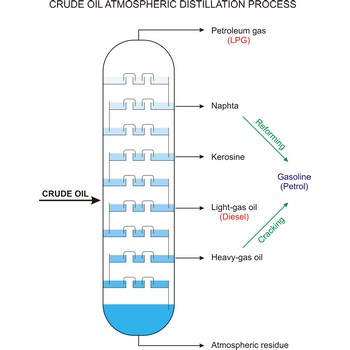
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
molar enthalpy of evaporation → molarna entalpija isparavanja
Molar enthalpy of evaporation (Δl gH) is a change of enthalpy during evaporation divided by molarity of a liquid, and is equal to the heat energy spent when the evaporation is conducted under constant pressure, Δl gH=Q.
Citing this page:
Generalic, Eni. "Pothlađena tekućina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table