chromatography → kromatografija
Chromatography is a method of separation of the components of a sample in which the components are distributed between two phases, one of which is stationary while the other moves. In gas chromatography, the gas moves over a liquid or solid stationary phase. In liquid chromatography, the liquid mixture moves through another liquid, a solid, or a gel. The mechanism of separation of components may be adsorption, differential solubility, ion-exchange, permeation, or other mechanisms.
covalent compound → kovalentni spoj
Covalent compound is a compound made of molecules - not ions, such as H2O, CH4, Cl2. The atoms in the compound are bound together by shared electrons. Also called a molecular compound.
effervescence → pjenušanje
Effervescence is the formation of gas bubbles in a liquid by a chemical reaction. An example of effervescence is the release of carbon dioxide which bubbles as a gas from the liquid when limestone chips, which are composed of calcium carbonate, are added to dilute hydrochloric acid.
elution → eluacija
Elution is the process of removing an adsorbed material (adsorbate) from an adsorbent with a liquid (eluent). The solution consisting of the adsorbate dissolved in the eluent is the eluate. Elution is the process used to wash components of a mixture through a chromatography column.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
colloid mill → koloidni mlin
Colloid mills are machines used to grind aggregates into very fine particles or to apply very high shearing within a fluid to produce colloid suspensions or emulsions in which the particle sizes are less than 1 micrometer. One type of colloid mill is called a disc mill, in which a mixture of a solid and liquid (or two liquids) is passed between two discs a small distance apart, which rotate very rapidly relative to each other. Applications of colloid mills occur in food processing, in paint manufacture, and in the pharmaceutical industry.
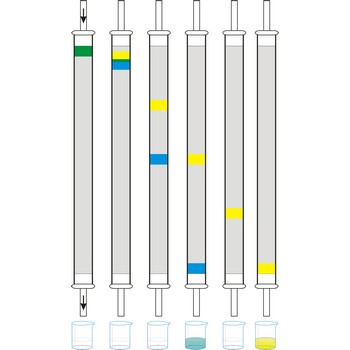
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
evaporation → isparavanje
Evaporation is the change of state of a liquid into a vapour at a temperature below the boiling point of the liquid.
Citing this page:
Generalic, Eni. "Pothlađena tekućina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table