dilution → razrjeđivanje
Dilution is the action of diluting or reducing the strength or concentration of a liquid, usually by the addition of water.
dissolved substance → otopljena tvar
Dissolved substance is a solid, liquid or gas matter dissolved in a solvent. Depending upon the particle size of dissolved substance, solutions differ in properties and can be divided into real solutions (diameter of particles is smaller than 1 nm), colloid solutions (diameter of particles is from 1 nm to 200 nm) and suspensions (diameter of particles is greater than 200 nm).
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
composition of ocean water → sastav oceanske vode
The proportions of the major constituents of ocean water are almost constant throughout the world. Salinity (total salt content) and the concentrations of individual chemical constituents in sea wateris given the units psu (practical salinity units). For most purposes one can assume that the new unit, psu, and the older unit, ‰, are synonymous.
The average composition of the ocean water is as shown on the following table.
| Constituent | Percentage of total salt |
|---|---|
| Chlorine | 55.3 % |
| Sodium | 30.8 % |
| Magnesium | 3.7 % |
| Sulphur | 2.6 % |
| Calcium | 1.2 % |
| Potassium | 1.1 % |
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
ebullioscopic constant → ebulioskopska konstanta
Ebullioscopic constant (Eb) is the constant that expresses the amount by which the boiling point Tb of a solvent is raised by a nondissociating solute, through the relation
where b is the molality of the solute.
fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
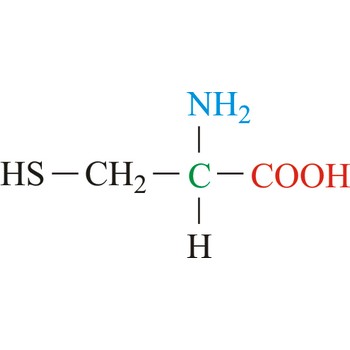
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
Citing this page:
Generalic, Eni. "Polarno otapalo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table