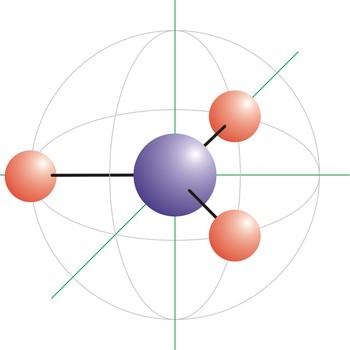
percentage composition of atom in the molecule → postotni sastav atoma u molekuli
Percentage composition of atom in the molecule is a structure of compound presented in the shape of a percentage of its mass, which comes from every element.
linear molecular geometry → linearna geometrija molekule
Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH2.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
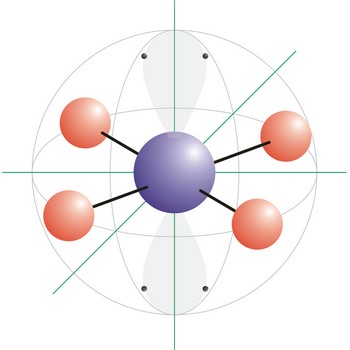
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
planary structure → planarna struktura molekule
Planary structure of molecule is a structure of molecule in which all atoms in the molecule lie in the same plane.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
square planar molecular geometry → kvadratna planarna geometrija molekule
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
Citing this page:
Generalic, Eni. "Polarna molekula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table