transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
tryglyceride → triglicerid
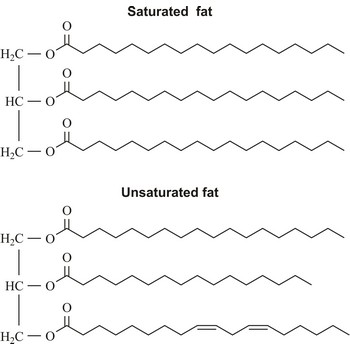
Triglyceride is an ester of glycerol and three fatty acids. It is the main constituent of vegetable oil and animal fats. The fatty acids attached to the glycerol can be the same or different. Natural fatty acids found in plants and animals are typically composed only of even numbers of carbon atoms (usually from 16 to 20) due to the way they are bio-synthesized from acetyl CoA.
tungsten → volfram
Tungsten was discovered by Fausto and Juan Jose de Elhuyar (Spain) in 1783. Named after the tungsten mineral wolframite. It is hard, steel-grey to white metal. Highest melting point of all metals. Resists oxygen, acids and alkalis. Tungsten occurs in the minerals scheelite (CaWO4) and wolframite [(Fe,Mn)WO4]. Made into filaments for vacuum tubes and electric lights. Also as contact points in cars. Tungsten carbide is extremely hard and is used for making cutting tools and abrasives.
tyrosine → tirozin
Tyrosine is hydrophobic amino acids with aromatic side chain. Tyrosine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tyrosine has special properties since its hydroxyl side chain may function as a powerful nucleophile in an enzyme active site (when ionized) and is a common site for phosphorylation in cell signaling cascades. Tyrosine absorbs ultraviolet radiation and contributes to the absorbance spectra of proteins. It is not essential (or semi-essential) to the human diet, since it is synthesized in the body from other metabolites.
- Abbreviations: Tyr, Y
- IUPAC name: 2-amino-3-(4-hydroxyphenyl)propanoic acid
- Molecular formula: C9H11NO3
- Molecular weight: 181.19 g/mol
U-tube manometer → U-manometar
U-tube manometer contains water or mercury in a U-shaped tube, and is usually used to measure gas pressure. One end of the U tube is exposed to the unknown pressure field (P) and the other end is connected to a reference pressure source (usually atmospheric pressure) (Pref), shown in the schematic below.
If fluid C is the atmosphere, fluid B is the liquid in the U tube (e.g. water or mercury), and fluid A is a gas, then we can assume that ρB >> ρA, ρC. The pressure contributed by the weight of gas within the U tube can therefore be neglected. The gage pressure of the gas can be approximated by,
ununbium → ununbij
Ununbium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in February 1996. The new element has not yet been officially named, but it is known as ununbium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Using the electromagnetic velocity filter SHIP, fusion-like residues of the reaction of 70Zn with enriched 208Pb targets were measured. Two chains of localized alpha-emitters were identified as originating with 277112 + 1n.
ununquadium → ununkvadij
The discovery of ununquadium was reported informally in January 1999 following experiments towards the end of December 1998 involving scientists at Dubna (Joint Institute for Nuclear Research) in Russia and the Lawrence Livermore National Laboratory, USA. The new element has not yet been officially named, but it is known as ununquadium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. Only few atoms of element 114 (289114) has ever been made (through a nuclear reaction involving fusing a calcium atom with a plutonium atom) isolation of an observable quantity has never been achieved.
unununium → unununij
Unununium was discovered by S. Hofmann et al. collaboration at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in December 1994. The new element has not yet been officially named, but it is known as unununium, according to the system designated by the IUPAC for naming new elements. It is synthetic radioactive metal. In bombardments of 209Bi targets with 64Ni using the velocity selector SHIP facility to discriminate in favor of the fused product, 272111 + 1n, three sets of localized alpha-decay chains were observed with position-sensitive detectors.
uranium → uranij
Uranium was discovered by Martin Heinrich Klaproth (Germany) in 1789. Named after the planet Uranus. It is silvery-white, dense, ductile, malleable, radioactive metal. Resists alkalis; tarnishes in air; attacked by steam and acids. Radiotoxic. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs.
Citing this page:
Generalic, Eni. "PloÅ¡no centrirana kubiÄna reÅ¡etka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table