state of matter → agregatno stanje
State of matter is one of the tree physical states in which matter can exist, i.e. solid, liquid or gas. Plasma is sometimes regarded as the fourth state of matter. By means of heating a solid substance will cross to liquid state at its melting point. If we heat up a liquid and beyond, at its boiling point it will cross to gaseous state - vapour.
liquid aggregate state → tekuće agregatno stanje
Liquids have a constant volume, but they do not have a constant shape. They assume the shape of a vessel they are in at the moment. It happens because molecules of liquids are still close enough to each other, the attracting forces still being very strong.
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
nascent state → nascentno stanje
Nascent state is an especially active state of an element in a moment when it is released from a compound during chemical reaction, e.g. nascent hydrogen.
change of state → promjena stanja
Change of state is a physical change which appears when a substance crosses from one state into another. This usually happens because of the change of energy of particles provoked by heating or cooling.
heat of sublimation → toplina sublimacije
Heat of sublimation or enthalpy of sublimation is the energy required to convert one mole of a substance from the solid to the gas state (sublimation) without the appearance of the liquid state.
condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
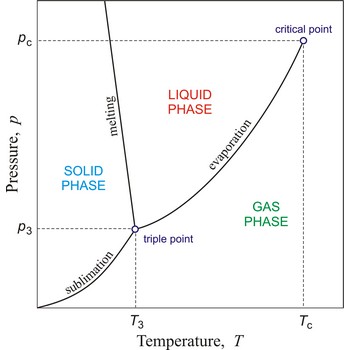
critical temperature → kritična temperatura
Critical temperature is the temperature of the liquid-vapour critical point, that is, the temperature above which a gas cannot be liquefied by an increase of pressure.
gas → plin
Gas is a state of matter, in which the mollecules move freely and consequently the entire mass tends to expand indefinitely, occupying the total volume of any vessel into which it is introduced. Gases follow, within considerable degree of fidelity, certain laws relating their conditions of pressure, volume and temperature. Gases mix freely with each other, and they can be liquefied through compression or temperature reduction.
equation of state → jednadžba stanja
Equation of state is an equation relating the pressure, volume, and temperature of a substance or system. Equation of state for ideal gas
where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
Citing this page:
Generalic, Eni. "Plinovito agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table