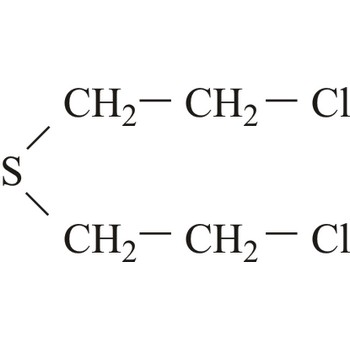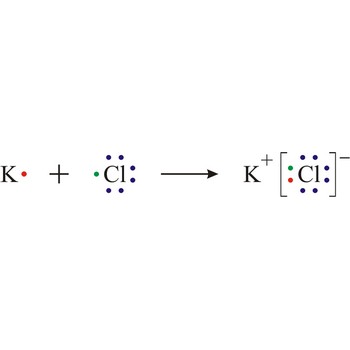gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
universal gas constant → univerzalna plinska konstanta
Universal gas constant R has the value of 8.314 472(15) J K-1 mol-1. It corresponds to the volume work performed by one mole of gas heated by 1 K at standard pressure.
ideal gas law → jednadžba stanja idealnog plina
The generalized ideal gas law is derived from a combination of the laws of Boyle and Charles. Ideal gas law is the equation of state
which defines an ideal gas, where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
Fajans’ rules → Fajansova pravila
Fajans’ rules, formulated by American chemist of Polish origin. Kazimierz Fajans (1887-1975), indicating the extent to which an ionic bond has covalent character caused by polarisation of the ions. Covalent character is more likely if:
1. the charge of the ions is high;
2. the positive ion is small or the negative ion is large;
3. the positive ion has an outer electron configuration that is not a noble- gas configuration.
groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
oxygen → kisik
Oxygen was discovered by Joseph Priestley (England) in 1774. The origin of the name comes from the Greek words oxy genes meaning acid and forming (acid former). It is colourless, odourless gas; pale blue liquid. Extremely reactive. Forms oxides with nearly all other elements except noble gases. It is the most abundant element in the earth’s crust and makes up almost 21 % of the atmosphere. Oxygen is obtained primarily from liquid air by fractional distillation. Small amounts are made in the laboratory by electrolysis of water. Used in steel making, welding and supporting life. Naturally occurring ozone (O3) in the upper atmosphere shields the earth from ultraviolet radiation.
period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
Citing this page:
Generalic, Eni. "Plemeniti plin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table