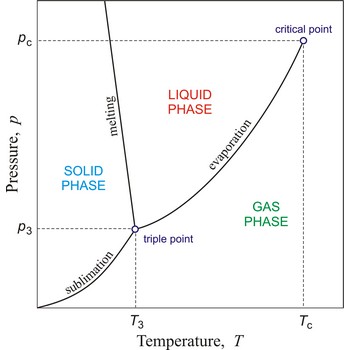
critical temperature → kritična temperatura
Critical temperature is the temperature of the liquid-vapour critical point, that is, the temperature above which a gas cannot be liquefied by an increase of pressure.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
molar enthalpy of melting → molarna entalpija taljenja
Molar enthalpy of melting (Δs lH) is a change of enthalpy during melting divided by the molarity of a solid matter, and is equal to the energy used when melting is conducted under constant pressure.
molar volume → molarni volumen
Molar volume is the volume occupied by substance per unit amount of substance. The volume of the gas at 0 °C and 101 325 Pa is 22.4 dm3mol-1.
normal boiling point → normalno vrelište
Normal boiling point is a temperature at which pressure of liquid vapour is 101 325 Pa.
osmometry → osmometrija
Osmometry is a determination of the average molecular weight of a dissolved substance from measurements of osmotic pressure.
osmosis → osmoza
Osmosis is the flow of a solvent in a system in which two solutions of different concentration are separated by a semipermeable membrane which cannot pass solute molecules. The solvent will flow from the side of lower concentration to that of higher concentration, thus tending to equalise the concentrations. The pressure that must be applied to the more concentrated side to stop the flow is called the osmotic pressure.
polymorphic transition → polimorfni prijelaz
Polymorphic transition is a reversible transition of a solid crystalline phase at a certain temperature and pressure to another phase of the same chemical composition with a different crystal structure. For examples, the transitions of quartz (SiO2) at 1 143 K to tridymite, and at 1 743 K to cristobalite.
Euler number → Eulerova značajka
Euler number (Eu) is a dimensionless quantity used in fluid mechanics, defined by
where p is pressure, ρ is density, and v is velocity.
Citing this page:
Generalic, Eni. "Parcijalni tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table