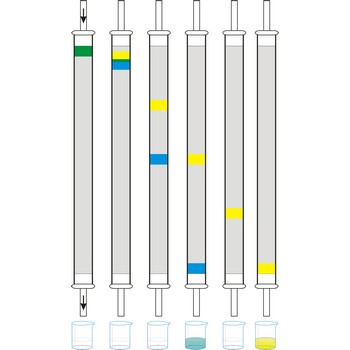
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
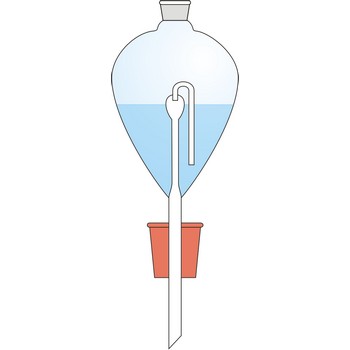
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
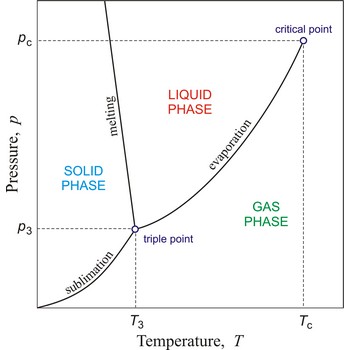
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
intensive property → intenzivno svojstvo
intensive property is a property that does not change when the amount of sample changes. Examples are density, pressure, temperature, colour.
isotonic solution → izotonična otopina
Isotonic solutions are the solutions that have equal osmotic pressure.
malleability → kovkost
Malleability is a property of something that can be worked or hammered or shaped under pressure without breaking.
millimetre of mercury → milimetar živina stupca
Millimetre of mercury (mmHg) is a non-SI unit of pressure, equal to 133.322 Pa. The name is generally considered interchangeable with torr.
molar enthalpy of evaporation → molarna entalpija isparavanja
Molar enthalpy of evaporation (Δl gH) is a change of enthalpy during evaporation divided by molarity of a liquid, and is equal to the heat energy spent when the evaporation is conducted under constant pressure, Δl gH=Q.
Citing this page:
Generalic, Eni. "Parcijalni tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table