chlorine → klor
Chlorine was discovered by Carl William Scheele (Sweden) in 1774. The origin of the name comes from the Greek word chloros meaning pale green. It is greenish-yellow, disagreeable gas with irritating odour. Gas is toxic and severe irritant by contact or inhalation. Never found in free form in nature. Commercial quantities of chlorine are produced by electrolysis of aqueous sodium chloride (NaCl) from seawater or brine from salt mines. Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC).
chromium → krom
Chromium was discovered by Louis-Nicholas Vauquelin (France) in 1797. The origin of the name comes from the Greek word chroma meaning colour. It is very hard, crystalline, steel-grey metal. The pure metal has a blue-white colour. It is hard, brittle and corrosion-resistant at normal temperatures. Hexavalent compounds toxic by skin contact. The most important chromium mineral is chromite [Fe,Mg(CrO4)]. Produced commercially by heating its ore in the presence of silicon or aluminium. Used to make stainless steel. It gives the colour to rubies and emeralds. Iron-nickel-chromium alloys in various percentages yield an incredible variety of the most important metals in modern technology.
electroorganic reaction → elektroorganske reakcije
Electroorganic reaction is an organic reaction produced in an electrolytic cell. Electroorganic reactions are used to synthesise compounds that are difficult to produce by conventional techniques. An example of an electroorganic reaction is Kolbe’s method of synthesising alkanes.
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
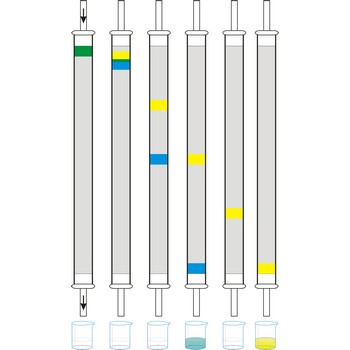
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
Citing this page:
Generalic, Eni. "Oxo compound." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table