conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
Grignard reagent → Grignardov reagens
Grignard reagents are organomagnesium halides, RMgX, having a carbon- magnesium bond (or their equilibrium mixtures in solution with R2Mg + MgX2).
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
immersion plating → galvaniziranje uranjanjem
Immersion plating involves depositing a metallic coating on a metal immersed in a liquid solution, without the aid of an external electric current. Also called dip plating.
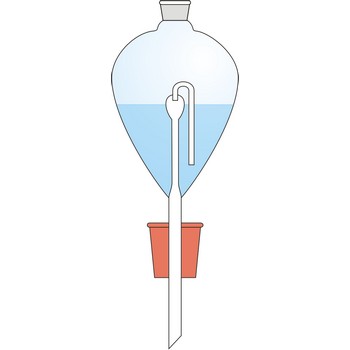
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
isomorphism → izomorfija
Isomorphism is the existence of two or more substances that have the same crystal structure, so that they form solid solutions.
manganometry → manganometrija
Manganometry is a quantitative oxidimetric method based on measurement of potassium permanganate (KMnO4) spent for an oxidation of the matter in question.
Citing this page:
Generalic, Eni. "Otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table