diffusion current → difuzijska struja
Diffusion current (id) is a current which is limited by the speed of particle diffusion in an electrolyte solution.
digestion → digeriranje
Digestion or precipitate ageing happens when freshly formed precipitate are left, usually at a higher temperature, in a solution from which it is precipitated. It results in cleaner and bigger particles.
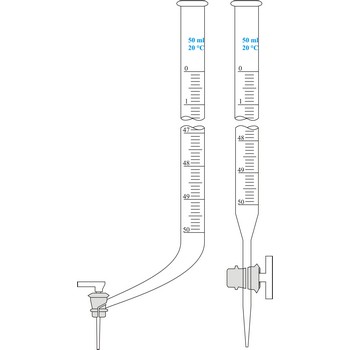
burette → bireta
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
dilution → razrjeđivanje
Dilution is the action of diluting or reducing the strength or concentration of a liquid, usually by the addition of water.
dissolved substance → otopljena tvar
Dissolved substance is a solid, liquid or gas matter dissolved in a solvent. Depending upon the particle size of dissolved substance, solutions differ in properties and can be divided into real solutions (diameter of particles is smaller than 1 nm), colloid solutions (diameter of particles is from 1 nm to 200 nm) and suspensions (diameter of particles is greater than 200 nm).
chlorine → klor
Chlorine was discovered by Carl William Scheele (Sweden) in 1774. The origin of the name comes from the Greek word chloros meaning pale green. It is greenish-yellow, disagreeable gas with irritating odour. Gas is toxic and severe irritant by contact or inhalation. Never found in free form in nature. Commercial quantities of chlorine are produced by electrolysis of aqueous sodium chloride (NaCl) from seawater or brine from salt mines. Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC).
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Donnan potential → Donnanov potencijal
Donnan potential is the electrical potential difference between two solutions separated by an ion-exchange membrane in the absence of any current flowing through the membrane
Citing this page:
Generalic, Eni. "Otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table