triols → trioli
Trihydric alcohols (i.e. Triols) are organic compounds containing three hydroxyl groups. The simplest trihydric alcohol is 1,2,3-propane-triol, CH2(OH)CH(OH)CH2(OH), which is also known as glycerol (from the Greek glykys meaning sweet) or glycerin. Glycerol is commercially produced by the hydrolysis of fats.
Glycerol is a by-product in the soap industry and is recovered by suitable means.
water → voda
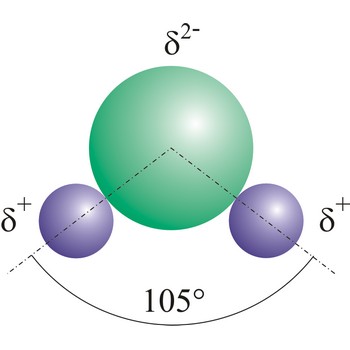
Water (H2O) (dihydrogen oxide) is a binary compound that occurs at room temperature as a clear colorless odorless tasteless liquid; freezes into ice below 0 °C and boils above 100 °C. Water is a chemical compound which is essential for living organisms and it is widely used as a solvent.
water gas → vodeni plin
Water gas (blue gas, synthesis gas) is a fuel gas used in industrial synthesis of organic chemicals, and in welding, glassmaking, and other high-temperature industrial applications. Water gas is made by passing steam over a bed of hot coal or coke. It mainly consists of carbon monoxide (CO) and hydrogen (H2), contaminated with small amounts of CO2, N2, CH4, and O2.
Winkler’s method → Winklerova metoda
Winkler’s method was once a common method used to determine the dissolved oxygen concentration by titration. Now rarely used due to the accuracy and low price of oxygen meters.
The water sample is first treated with excess manganese(II) sulfate solution and then with an alkaline solution of potassium iodide. The Mn(OH)2 initially formed reacts with the dissolved oxygen. The amount of MnO(OH)2 formed is determined by reaction with iodide ion in acidic solution. The iodine formed may be titrated against standard thiosulfate solution, using starch as an indicator.
Wohler’s synthesis → Wohlerova sinteza
Wöhler’s synthesis is a synthesis of urea performed by the German chemist Friedrich Wöhler (1800-1882) in 1828. He discovered that urea (CO(NH2)2) was formed when a solution of ammonium isocyanate (NH4NCO) was evaporated. At the time it was believed that organic substances such as urea could only be made by living organisms, and its production from an inorganic compound was a notable discovery.
Dipeptide → Dipeptid
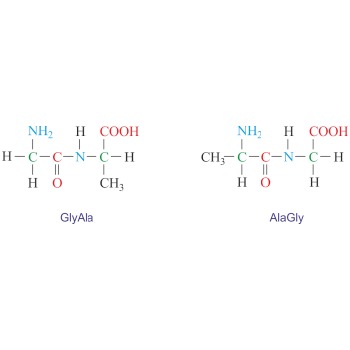
Dipeptide is an organic compound formed when two amino acids are joined by a peptide bond. Depending on which groups of amino acids are involved in the peptide bond four dipeptides can be formed from two different amino acids. For example, glycine (Gly) and alanine (Ala) can give two symmetrical dipeptides (GlyGly and AlaAla) and two unsymmetrical dipeptides (GlyAla and AlaGly). The naming is done by reading the sequence from the N-terminus to the C-terminus.
Citing this page:
Generalic, Eni. "Organsko otapalo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table