acrylic acid → akrilna kiselina
Acrylic acid (propenoic acid) is a colourless liquid, smelling like acetic acid. It can be formed by acrolein oxidation. It readily polymerizes and is used in the manufacture of acrylic resins, transparent plastic materials (organic glass).
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
aldehydes → aldehidi
Aldehydes are a broad class of organic compounds having the generic formula RCHO, and characterized by an unsaturated carbonyl group (C=O). They are formed from alcohols by either dehydrogenation or oxidation. Their chemical derivation is indicated by the name al(cohol) + dehyd(rogenation). An example of these distinct aromatic compounds is formaldehyde.
alicyclic → aliciklički spojevi
Alicyclic compounds are aliphatic compounds with a ring of atoms. They have CnH2n general formula (e.g. cyclohexane C6H12).
alkaloid → alkaloid
Alkaloids are basic nitrogen organic compounds (mostly heterocyclic) derived from plants and having diverse pharmacological properties. Alkaloids include morphine, cocaine, atropine, quinine, and caffeine, most of which are used in medicine as analgesics or anaesthetics. Some alkaloids are poisonous, e.g. strychnine and coniine, and colchicine inhibit cell division.
activated charcoal → aktivni ugljen
Activated charcoal or activated carbon is charcoal that has been activated for adsorption by steaming or by heating in a vacuum. Charcoal is obtained by burning wood, nutshells, coconut husks or other materials. Charcoal becomes activated by heating it with steam to approximately 1000 °C in the absence of oxygen.
The chemical nature of amorphous carbon, combined with a high surface area makes it an ideal medium for the adsorption of organic chemicals. A single gram of such material can have 400 m2 to 1 200 m2 square meters of surface area. Activated charcoal is widely used to decolorize liquids, recover solvents, and remove toxins from water and air.
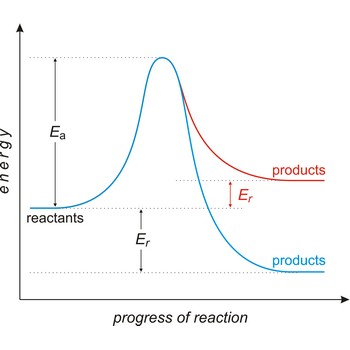
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
Citing this page:
Generalic, Eni. "Organska kemija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table