copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
corrosion → korozija
Corrosion is a harmful and undesirable construction material consumption by the chemical activity of its surroundings. Corrosion concept refers to metal and nonmetal construction materials, but it is usually used for metals, Corrosion of metal, according to the mechanism process, is divided into chemical (corrosion in nonelectrolytes) and electrochemical (corrosion in electrolytes).
Chemical corrosion appears by direct action of molecule of some element or compound on metal, thus directly creating corrosion products.
Electrochemical corrosion of metals occurs in electrolytes, so reduction of metal atom into free cation appears which by secondary processes gives molecules of compound which are considered a corrosion product.
cysteine → cistein
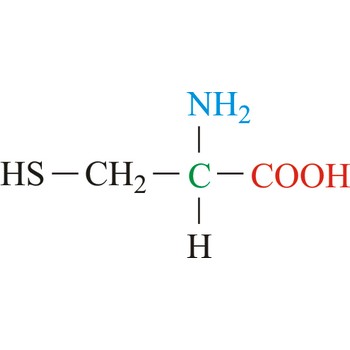
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
decomposition → raspadanje
Decomposition occurs when chemical compounds are broken up into simple molecules, and even as far as their original elements. These processes are normally irreversible. An example of decomposition is when ammonium nitrate is heated. This produces nitrous oxide and water which are unable to recombine.
reactive metal → reaktivni metal
Reactive metals are metals that readily combine with oxygen at elevated temperatures to form very stable oxides, for example titanium, zirconium, and beryllium. Reactive metals may also become embrittled by the interstitial absorption of oxygen, hydrogen, and nitrogen.
thermit welding → termitno zavarivanje
Thermit welding is a group of welding processes in which fusion is produced by heating with superheated liquid metal resulting from a chemical reaction between a metal oxide and aluminium.
Zimmermann-Reinhardt’s reagent → Zimmermann-Reinhardtov reagens
Zimmermann-Reinhardt’s reagent is a mixture of manganese(II) sulphate, sulphuric acid and phosphorus acid. It is used for preventing oxidation of chloride ion while titrating iron(II) ion with permanganate solution.
Citing this page:
Generalic, Eni. "Oksim." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table