arsenic → arsen
Arsenic was discovered by Albertus Magnus (Germany) in 1250. The origin of the name comes from the Greek word arsenikon meaning yellow orpiment. It is steel-grey, brittle semi-metal. Resists water, acids and alkalis. Tarnishes in air, burns in oxygen. Highly toxic by inhalation or ingestion. Arsenic is found in mispickel (arsenopyrite). Many of its compounds are deadly poison and used as weed killer and rat poison. Used in semiconductors. Some compounds, called arsenides, are used in the manufacture of paints, wallpapers and ceramics.
autocatalysis → autokataliza
Autocatalysis is a reaction in which its product can act as a catalyst. Oxalate oxidation with permanganate in an acid solution is a slow reaction
Mn2+-ions catalyse this reaction. When enough Mn2+-ions are created, the reaction occurs instantly.
barrier film → barijerni film
Barrier film is a thin, continuous, non-porous, electrically insulating film on metal surfaces (usually comprised of oxides).
basic Bessemer process → bazni Bessemerov proces
Basic Bessemer process is the process of steelmaking in a way that steel, melted iron and limestone are put in the blast-furnace after which the metal oxygen is released in gushes in order to oxidise the impurities.
blanching → blanširanje
1. Blanching is a heat treatment of foodstuffs to partially or completely inactivate the naturally occurring enzymes prior to freezing.
2. Blanching is a washing process for coins cleaning. The black surface layer of cupric oxide is removing by dipping the coins in hot dilute sulphuric acid (w(H2SO4) = 10 %).
Boudouard’s equilibrium → Boudouardova ravnoteža
Boudouard’s equilibrium is established when carbon dioxide reacts with carbon. Because of reactions endothermity the temperature increase shifts the reaction rightwards and the temperature reduction leftwards.
base → baza
Historically, base is a substance that yields an OH - ion when it dissociates in solution, resulting in a pH>7. In the Brønsted definition, a base is a substance capable of accepting a proton in any type of reaction. The more general definition, due to G.N. Lewis, classifies any chemical species capable of donating an electron pair as a base. Typically, bases are metal oxides, hydroxides, or compounds (such as ammonia) that give hydroxide ions in aqueous solution.
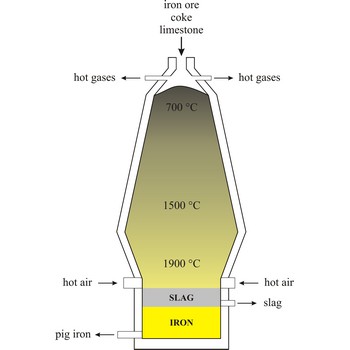
blast furnace → visoka peć
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
bromine → brom
Bromine was discovered by Antoine J. Balard (France) in 1826. The origin of the name comes from the Greek word bromos meaning stench. It is reddish-brown liquid with suffocating, irritating fumes. Gives off poisonous vapour. Causes severe burns. Oxidizer. Bromine occurs in compounds in sea water. It was once used in large quantities to make a compound that removed lead compound build up in engines burning leaded gasoline. Now it is primarily used in dyes, disinfectants and photographic chemicals.
Citing this page:
Generalic, Eni. "Oksim." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table