vanadium → vanadij
Vanadium was discovered by A. M. del Rio (Spain) in 1801 and rediscovered by Nils Sefstrom (Sweden) in 1830. Named after Vanadis, the goddess of beauty in Scandinavian mythology. It is soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Exposed surfaces form oxide coating. Reacts with concentrated acids. Vanadium is found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl] and carnotite [K2(UO2)2(VO4)2·3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere. It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and fixer-fixer.
Volta, Alessandro → Volta, Alessandro
The Italian physicist Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) was the inventor of the voltaic pile, the first electric battery (1800). In 1775 he invented the electrophorus, a device that, once electrically charged by having been rubbed, could transfer charge to other objects. Between 1776 and 1778, Volta discovered and isolated methane gas (CH4). The electrical unit known as the volt was named in his honor.
water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
work → rad
Work is the energy required to move an object against an opposing force. Work is usually expressed as a force times a displacement.
When a constant force F acts on a point-like object while the object moves through a displacement s, the force does work W on the object. If force and displacement are at a constant angle Θ to each other, the work is expressed by the scalar product of these two vectors:
When the force F on a point-like object is not constant that is, it depends on the position of the object, the work done by force while object moves from initial position with coordinates (xi, yi, zi) to final position with coordinates (xf, yf, zf)is given by expression:
Where Fx, Fy and Fz are scalar components of the force.
SI unit for work is joule (J); 1 J = 1 Nm = 1 kg m2 s-2. The electron-volt (eV) is commonly used in atomic and nuclear physics.
X-ray diffraction → rendgenska difrakcija
The regular array of atoms in a crystal is a three-dimensional diffraction grating for short-wavelength waves such as X-rays. The atoms are arranged in planes with interplanar spacing d. Diffraction maxima occur in the incident direction of the wave, measured from the surface of a plane of atoms, and the wavelength λ of the radiation satisfy Braggs’s law:
ytterbium → iterbij
Ytterbium was discovered by Jean de Marignac (France) in 1878. Named after Ytterby, a village in Sweden. It is silvery, lustrous, malleable and ductile metal. Oxidizes slowly in air. Reacts with water. Flammable dust. Ytterbium is found in minerals such as yttria, monazite, gadolinite and xenotime. Used in metallurgical and chemical experiments.
yttrium → itrij
Yttrium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is silvery, ductile, fairly reactive metal. Exposed surfaces form oxide film. Easily combustible, reacts with oxygen in water to release hydrogen. Yttrium is found in minerals such as monazite, xenotime and yttria. Combined with europium to make red phosphors for colour TV’s. Yttrium oxide and iron oxide combine to form a crystal garnet used in radar.
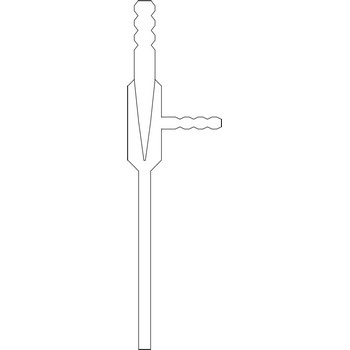
water jet vacuum pump → vodena sisaljka
The water jet vacuum pump or vacuum aspirator is one of the most popular devices that produces vacuum in laboratories. The rapid flow of water through the device creates a vacuum in a side-arm that is connected to a vacuum application such a Buchner flask. The water jet vacuum pump creates a vacuum by means of Venturi effect named after the Italian physicist Giovanni Battista Venturi (1746–1822). The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. Water jet pumps are manufactured of glass, plastic or metal, depending on the conditions in which they are used.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
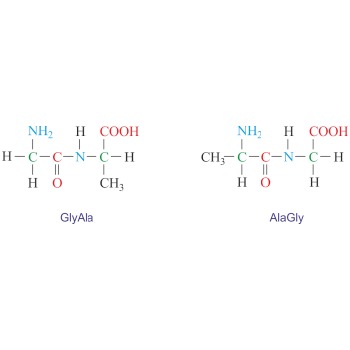
Dipeptide → Dipeptid
Dipeptide is an organic compound formed when two amino acids are joined by a peptide bond. Depending on which groups of amino acids are involved in the peptide bond four dipeptides can be formed from two different amino acids. For example, glycine (Gly) and alanine (Ala) can give two symmetrical dipeptides (GlyGly and AlaAla) and two unsymmetrical dipeptides (GlyAla and AlaGly). The naming is done by reading the sequence from the N-terminus to the C-terminus.
Citing this page:
Generalic, Eni. "Nginx server_name regular expression." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table