Millon’s reaction → Millonova reakcija
Millon’s reaction is used for testing proteins by the appearance of red colour which the proteins give by reacting with a solution of mercury in nitric acid.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
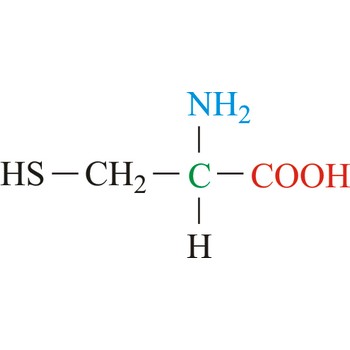
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
neptunium → neptunij
Neptunium was discovered by Edwin M. McMillan and P. H. Abelson (USA) in 1940. Named after the planet Neptune. It is rare, silvery radioactive metal. Resists alkalis; reacts with oxygen and acids. Attacked by steam. Radiotoxic. Neptunium was produced by bombarding uranium with slow neutrons.
neutral substance → neutralna tvar
Neutral substance is a substance that shows no acid or base properties, has an equal number of hydrogen and hydroxyl ions and does not change the colour of litmus-paper.
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
neutralisation → neutralizacija
Neutralisation is the process in which an acid reacts with a base to form a salt and water.
Citing this page:
Generalic, Eni. "Nezasićena masna kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table