dysprosium → disprozij
Dysprosium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1886. The origin of the name comes from the Greek word dysprositos meaning hard to obtain. It is soft, lustrous, silvery metal. Reacts with oxygen. Reacts rapidly with water; dissolves in acids. Metal ignites and burns readily. Reductant. Dysprosium usually found with erbium, holmium and other rare earths in some minerals such as monazite sand. Dysprosium uses are limited to the experimental and esoteric. Some isotopes of dysprosium are effective absorbers of thermal neutrons and are being considered for use in the control rods in nuclear reactors.
hafnium → hafnij
Hafnium was discovered by Dirk Coster (Denmark) and Georg Karl von Hevesy (Hungary) in 1923. The origin of the name comes from the Latin name Hafnia meaning Copenhagen. It is silvery, ductile metal. Exposed surfaces form oxide film. Resists alkalis and acids (except HF). Toxic. Metal ignites and burns readily. Hafnium is obtained from mineral zircon or baddeleyite. Used in reactor control rods because of its ability to absorb neutrons.
neptunium → neptunij
Neptunium was discovered by Edwin M. McMillan and P. H. Abelson (USA) in 1940. Named after the planet Neptune. It is rare, silvery radioactive metal. Resists alkalis; reacts with oxygen and acids. Attacked by steam. Radiotoxic. Neptunium was produced by bombarding uranium with slow neutrons.
heavy water → teška voda
Water molecules are composed of two hydrogen atoms and one oxygen atom (H2O). If the hydrogen atoms of a water molecule are replaced by deuterium atoms, the result is heavy water (D2O). Deuterium differs from hydrogen by having one neutron in the nucleus of the atom. There is approx. one part in 5000 D2O in normal water and it can be concentrated by electrolysis. Heavy water has a higher boiling point (101.4 °C) and melts at 3.6 °C. Heavy water is 20/18=1.11 times heavier than ordinary water.
nuclear reactor → nuklearni reaktor
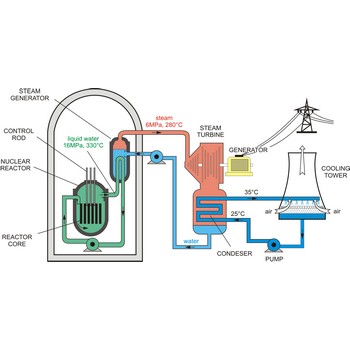
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
radiation damage → radijacijsko oštećenje
Radiation damage is a general term for the alteration of properties of a material arising from exposure to ionising radiation (penetrating radiation), such as X-rays, γ-rays, neutrons, heavy-particle radiation, or fission fragments in the nuclear fuel material.
subatomic particles → subatomske čestice
Subatomic particles are the constituent parts of the atom, such as the electron, proton, neutron, etc.
Citing this page:
Generalic, Eni. "Neutron." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table