enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
extraction → ekstrakcija
Extraction is the separation of a component from its mixture by selective solubility. When a solution of one substance in one solvent is brought in with another solvent dissolved substance will distribute between the two solutants because of different solubility. Extraction is an efficient and fast method used for separating and concentrating matters. Extraction is best done several times in a succession, with smaller amount of solvent in it the matter is better dissolved. For example, caffeine can be separated from coffee beans by washing the beans with supercritical fluid carbon dioxide; the caffeine dissolves in the carbon dioxide, but flavour compounds do not. Vanillin can be extracted from vanilla beans by shaking the beans with an organic solvent, like ethanol.
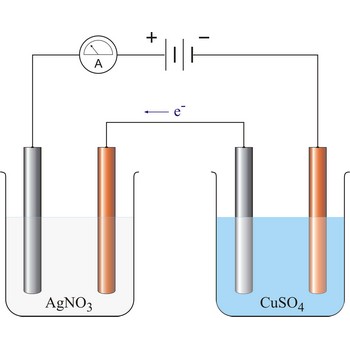
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
flash point → temperatura zapaljenja
Flash point is the lowest temperature at which a liquid or volatile solid gives off vapour sufficient to form an ignitable mixture with the air near the surface of the liquid or within the test vessel (NFPA).
ferromagnetism → feromagnetizam
Ferromagnetism is a type of magnetism in which the magnetic moments of atoms in a solid are aligned within domains which can in turn be aligned with each other by a weak magnetic field. The total magnetic moment of a sample of the substance is the vector sum of the magnetic moments of the component domains. In an unmagnetized piece of ferromagnetic material the magnetic moments of the domains themselves are not aligned; when an external field is applied those domains that are aligned with the field increase in size at the expense of the others. Ferromagnetic materials can retain their magnetisation when the external field is removed, as long as the temperature is below a critical value, the Curie temperature. They are characterised by a large positive magnetic susceptibility.
filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
flotation → flotacija
Flotation is a procedure in which hydrophobic solid substances are separated from hydrophilic one using bubbles of air. If air is blow through a suspension, in which substances promoting easier creation of foam are added, bubbles of air are created which stick to the hydrophobic matter and carry it out to the surface.
foam → pjena
Foams are dispersions of gases in liquids or solids. The gas globule may be of any size, from colloidal to macroscopic, as in soap bubbles. Bakers’ bread and sponge rubber are examples of solid foams. Typical liquid foams are those used in fire-fighting, shaving creams, etc. Foams made by mechanical incorporation of air are widely used in the food industry (e.g. whipped cream, egg white, ice cream, etc.). Foams can be stabilized by surfactants.
glass → staklo
Glass is a brittle transparent solid with the molecular structure of a liquid. It is made by fusing together sand (SiO2), soda (Na2CO3), and lime (CaCO3) with small quantities other compounds. It is used for window panes and mirrors, for articles of table and culinary use, for lenses, and various articles of ornament.
Citing this page:
Generalic, Eni. "Neutralna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table