coulometer → kulometar
Coulometer is a type of electrolysis cell which is used for measuring the quantity of one element released during electrolysis.
cubic close-packed structure → kubična gusta slagalina
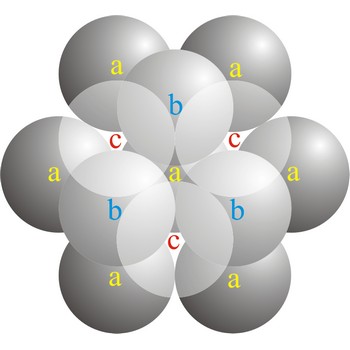
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
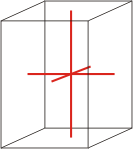 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
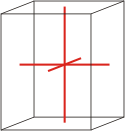 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
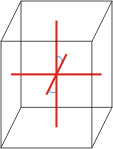 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
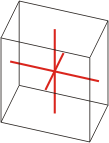 |
a≠b≠c α≠β≠γ≠90° |
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
face-centered orthorhombic lattice → plošno centrirana ortorompska rešetka
Face-centered orthorhombic lattice (orthorhombic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
hardness → tvrdoća
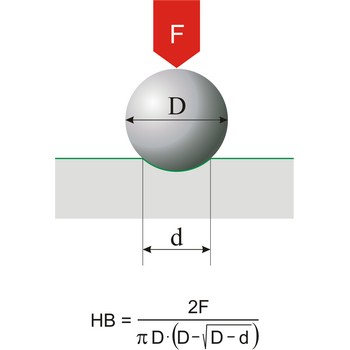
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
hexagonal close-packed structure → heksagonska gusta slagalina
In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer (b) contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed. The hexagonal close packed structure can be made by piling layers in the a-b-a-b-a-b... sequence.
Citing this page:
Generalic, Eni. "Nehrđajući čelik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table