electroanalytical chemistry → elektroanalitička kemija
Electroanalytical chemistry chemistry is the application of electrochemical cells and electrochemical techniques for chemical analysis. The analyte is dissolved in the electrolyte of the cell, and one can perform either qualitative analysis (determination of the type of constituents present) or quantitative analysis (determination of the amount of a given constituent).
bomb calorimeter → kalorimetrijska bomba
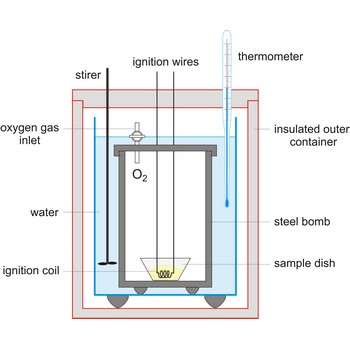
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
Brinell hardness → Brinellova tvrdoća
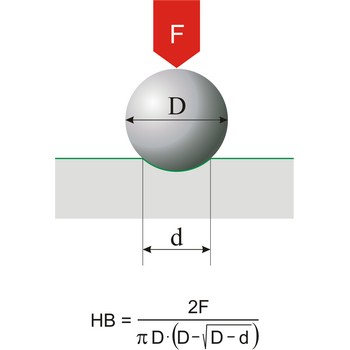
Brinell hardness is a scale for measuring the hardness of metals introduced around 1900 by Swedish metallurgist Johan Brinell (1849-1925). A small chromium steel ball is pressed into the surface of the metal by a load of known weight. The loading force is in the range of 300 N to 30 000 N. The ratio of the mass of the load in kilograms to the area of the depression formed in square millimetres is the Brinell Hardness Number.
caesium → cezij
Caesium was discovered by Robert Bunsen and Gustav Kirchhoff (Germany) in 1860. The origin of the name comes from the Latin word caesius meaning sky blue or heavenly blue. It is very soft, light grey, ductile metal. Reacts readily with oxygen. Reacts explosively with water. Caesium is found in pollucite [(Cs4Al4Si9O26)·H2O] and as trace in lepidolite. Used as a ’getter’ to remove air traces in vacuum and cathode-ray tubes. Also used in producing photoelectric devices and atomic clocks. Since it ionises readily, it is used as an ion rocket motor propellant.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
electroorganic reaction → elektroorganske reakcije
Electroorganic reaction is an organic reaction produced in an electrolytic cell. Electroorganic reactions are used to synthesise compounds that are difficult to produce by conventional techniques. An example of an electroorganic reaction is Kolbe’s method of synthesising alkanes.
galvanostat → galvanostat
Galvanostat is an electronic instrument that controls the current through an electrochemical cell at a preset value, as long as the needed cell voltage and current do not exceed the compliance limits of the galvanostat.
groundbed → zemljana anoda
Groundbed is a buried item, such as junk steel or graphite rods, that serves as the anode for the cathodic protection of pipelines or other buried structures.
lattice constants → konstante kristalne rešetke
Lattice constants are parameters specifying the dimensions of a unit cell in a crystal lattice, specifically the lengths of the cell edges and the angles between them.
Citing this page:
Generalic, Eni. "Nehrđajući čelik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table