atom marking → markiranje atoma
Atom marking is a process of infusing radioactive isotopes in live organism, with the purpose of revealing a way, diffusion, or a role of certain substance.
autoignition temperature → temperatura samozapaljenja
Autoignition temperature is the minimum temperature required to initiate or cause self-sustained combustion in any substance in the absence of a spark or flame. This varies with the test method.
allotropic modification → alotropska modifikacija
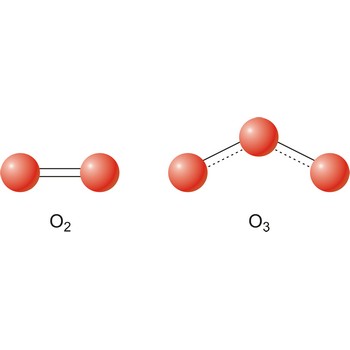
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
amount concentration → količinska koncentracija
Amount concentration (also called molar concentration and in older literature molarity) is the amount of a given substance in a stated unit of a mixture, solution, or ore. The common unit is mole per cubic decimetre (moldm−3) or mole per litre (molL-1) sometimes denoted by M.
The concentration of an atom, ion, or molecule in a solution may be symbolised by the use of square brackets, as [Ca2+].
auxochrome → auksokromna grupa
Auxochrome is a group or substructure in a molecule that influences the intensity of absorption of the molecule.
Avogadro constant → Avogadrova konstanta
Avogadro constant (NA or L) is the number of elementary entities in one mole of a substance.
It has the value (6.022 045±0.000 031)×1023 mol-1.
basic Bessemer process → bazni Bessemerov proces
Basic Bessemer process is the process of steelmaking in a way that steel, melted iron and limestone are put in the blast-furnace after which the metal oxygen is released in gushes in order to oxidise the impurities.
amount fraction → količinski udio
Amount fraction, xA, (y for gaseous mixtures) is the ratio of the amount of substance (number of moles) of substance A to the total amount of substance in a mixture.
Citing this page:
Generalic, Eni. "Nečista tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table