chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
acylaction reaction → reakcije aciliranja
Acylaction reaction involves the introduction of an acyl group (RCO-) into a compound. An alkyl halide is reacted with an alcohol or a carboxylic acid anhydride e.g.
The introduction of an acetyl group (CH3CO-) is acetylation, a process used for protecting -OH groups in organic synthesis.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
Acheson process → Achesonov proces
Acheson process is an industrial process to synthesize graphite and silicon carbide (carborundum), named after its inventor the American chemist Edward Goodrich Acheson (1856-1931). In this process, a solid-state reaction between pure silica sand (SiO2) and petroleum coke (C) at very high temperature (more than 2500 °C) leads to the formation of silicon carbide under the general reaction:
While studying the effects of high temperature on carborundum, Acheson had found that silicon vaporizes at about 4150 °C, leaving behind graphitic carbon.
acid dissociation constant → konstanta disocijacije kiseline
Acid dissociation constant (Ka) is the equilibrium constant for the dissociation of an acid HA through the reaction
The quantity pKa = -log Ka is often used to express the acid dissociation constant.
acid rain → kisela kiša
Acid rain is rainwater that shows acid reaction because of nitrogen and sulphur oxides absorption. It is generated mainly by industrial pollutions.
activated complex → aktivirani kompleks
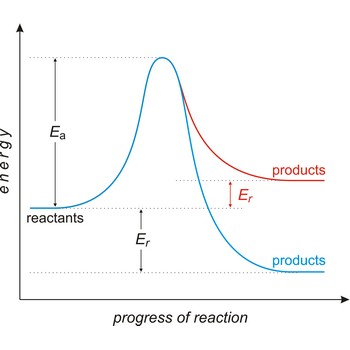
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
Citing this page:
Generalic, Eni. "Napredna reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table