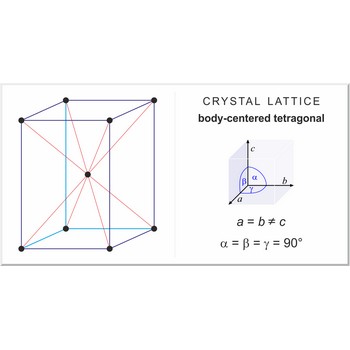
body-centered tetragonal lattice → prostorno centrirana tetragonska rešetka
Body-centered tetragonal lattice (tetragonal-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
face-centered orthorhombic lattice → plošno centrirana ortorompska rešetka
Face-centered orthorhombic lattice (orthorhombic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
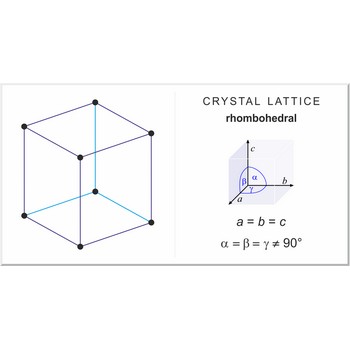
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
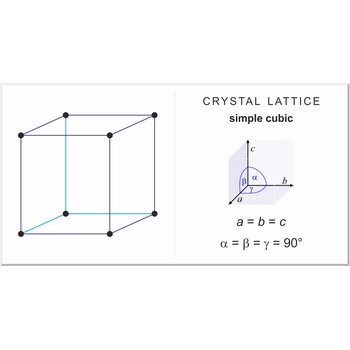
simple cubic lattice → jednostavna kubična rešetka
Simple or primitive cubic lattice (sc or cubic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a = b = c and interaxial angels α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the sc structures the spheres fill 52 % of the volume. The number of atoms in a unit cell is one (8×1/8 = 1). This is only one metal (α-polonium) that have the sc lattice.
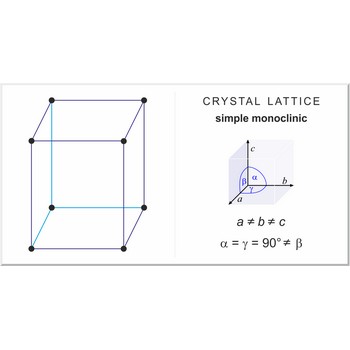
simple monoclinic lattice → jednostavna monoklinska rešetka
Simple or primitive monoclinic lattice (monoclinic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=γ=90°≠β.
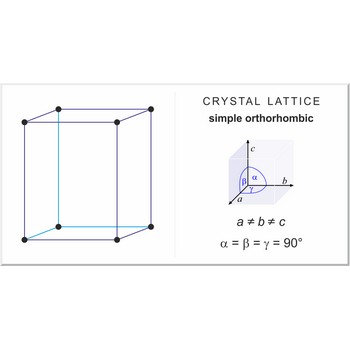
simple orthorhombic lattice → jednostavna ortorompska rešetka
Simple or primitive orthorhombic lattice (orthorhombic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
simple tetragonal lattice → jednostavna tetragonska rešetka
Simple or primitive tetragonal lattice (tetragonal-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
Citing this page:
Generalic, Eni. "Molekularna rešetka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table