sand filtration → pješčana filtracija
Sand filtration is a frequently used and very robust method of removing suspended solids from water. The filtration medium consists of a multiple layer of sand with a variety in size and specific gravity. Sand filters can be supplied in different sizes and materials, both hand operated and fully automatically.
heat capacity → toplinski kapacitet
Heat capacity is defined in general as dQ/dT, where dQ is the amount of heat that must be added to a system to increase its temperature by a small amount dT. The heat capacity at a constant pressure is Cp = (∂H/∂T)p; that at a constant volume is CV = (∂E/∂T)V, where H is enthalpy, E is internal energy, p is pressure, V is volume, and T is temperature. An upper case C normally indicates the molar heat capacity, while a lower case c is used for the specific (per unit mass) heat capacity.
Henry’s law → Henryjev zakon
Henry’s law was discovered in 1801 by the British chemist William Henry (1775-1836). At a constant temperature the mass of gas dissolved in a liquid at equilibrium is proportional to the partial pressure of the gas. It applies only to gases that do not react with the solvent.
where pi is the partial pressure of component i above the solution, xi is its mole fraction in the solution, and Kx is the Henry’s law constant (a characteristic of the given gas and solvent, as well as the temperature).
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
ideal gas law → jednadžba stanja idealnog plina
The generalized ideal gas law is derived from a combination of the laws of Boyle and Charles. Ideal gas law is the equation of state
which defines an ideal gas, where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
Strouhal number → Strouhalova značajka
Strouhal number (Sr) is a dimensionless quantity used in fluid mechanics, defined by
where l is length, f is frequency, and v is velocity.
temperature → temperatura
Temperature is a measure to the average kinetic energy of its molecules. The SI unit in which thermodynamic temperature is expressed is the kelvin (K).
inertia → inercija
Inertia is an expression for the tendency of all bodies to resist motion, or to continue in motion if already moving. If a body undergoes linear motion (translation), inertia corresponds to the mass of the body. In order to express inertia of a rotating body, the so-called rotational inertia is defined as suitable physical quantity. A bowling ball has more inertia than a tennis ball, due to its higher mass.
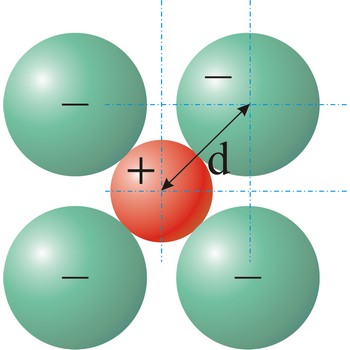
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
mass fraction → maseni udio
Mass fraction (wA) is the ratio of the mass of substance A to the total mass of a mixture.
Citing this page:
Generalic, Eni. "Molarna veličina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table