Winkler’s method → Winklerova metoda
Winkler’s method was once a common method used to determine the dissolved oxygen concentration by titration. Now rarely used due to the accuracy and low price of oxygen meters.
The water sample is first treated with excess manganese(II) sulfate solution and then with an alkaline solution of potassium iodide. The Mn(OH)2 initially formed reacts with the dissolved oxygen. The amount of MnO(OH)2 formed is determined by reaction with iodide ion in acidic solution. The iodine formed may be titrated against standard thiosulfate solution, using starch as an indicator.
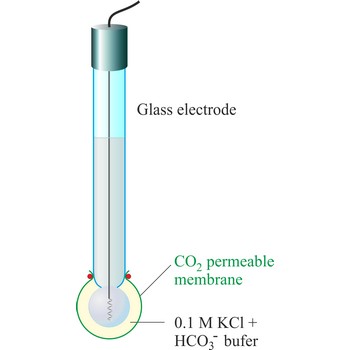
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
Citing this page:
Generalic, Eni. "Molarna koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table