burette → bireta
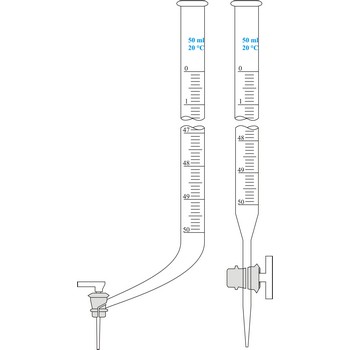
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
centrifuge → centrifuga
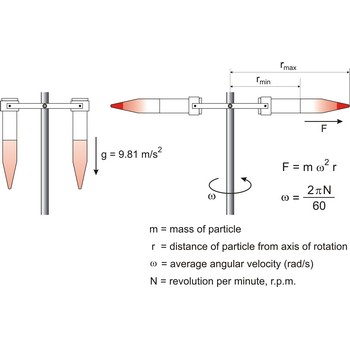
Centrifuge is a device in which solid or liquid particles of different densities are separated by rotating them in a tube in a horizontal circle. The dense particles tend to move along the length of the tube to a greater radius of rotation, displacing the lighter particles to the other end.
cetane number → cetanski broj
Cetane number is a measure of the ignition quality of diesel fuel. It denotes the volume fraction of cetane (C16H34) in a combustible mixture (containing cetane and 1-methylnapthalene) whose ignition characteristics match those of the diesel fuel being tested. Cetane is a collection of un-branched open chain alkane molecule that ignites very easily under compression, so it was assigned a cetane number of 100, while alpha-methyl naphthalene was assigned a cetane number of 0.
close-packed structure → gusta slagalina
Close packing is the packing of spheres so as to occupy the minimum amount of space. The name close packed refers to the packing efficiency of 74.05 %. There are two types of close packing: hexagonal and cubic. One layer, with atoms centered on sites labeled a. Two layers, with the atoms of the second layer centered on sites labeled b. The third layer can be placed on the sites labeled c (giving cubic close-packing) or over those marked a (giving hexagonal close-packing).
natural gas → prirodni plin
Natural gas is a naturally occurring mixture of gaseous hydrocarbons. The approximate composition of natural gas is 85 % methane, 10 % ethane, 3 % propane, with lesser amounts of butane, and other higher alkanes. Natural gas is used as a fuel and for the manufacture of chemicals.
nautical mile → morska milja
Nautical mile is a legal international unit of length temporarily maintained with the SI. It is still used in navigation (merchant marine, aviation). It is equal to the length of an arc of one minute measured at a latitude of N 45° (mile = 1852 m). The international nautical mile has been taken equal to the nautical mile.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
osmometry → osmometrija
Osmometry is a determination of the average molecular weight of a dissolved substance from measurements of osmotic pressure.
Citing this page:
Generalic, Eni. "Mjed." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table