rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
salt fog test → ispitivanje u slanoj komori
Salt fog test is an accelerated corrosion test in which specimens are exposed to a fine mist of a solution usually containing sodium chloride (typically 5 %). Other contaminants can be added according to desired conditions. It is mainly used to determine the effectiveness of material finishes and protective coatings on materials. Salt-fog testing is also used to determine the effects of salt deposits on the electrical functions of electronic assemblies.
sedimentation → sedimentiranje
Sedimentation is a process of separating specifically heavier, suspended matter, than the solution is. Solid matter settles on the bottom of the vessel and the liquid above it is poured off. The settling zone is the largest portion of the sedimentation basin. This zone provides the calm area necessary for the suspended particles to settle. The sludge zone, located at the bottom of the tank, provides a storage area for the sludge before it is removed for additional treatment or disposal.
solubility → topljivost
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a specific temperature. Generally, for a solid in a liquid, solubility increases with temperature; for a gas, solubility decreases. Common measures of solubility include the mass of solute per unit mass of solution (mass fraction), mole fraction of solute, molality, molarity, and others.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
sugar → šećer
Sugar is any of a group of water-soluble carbohydrates of relatively low molecular weight and typically having a sweet taste. The group comprises mainly monosaccharides (glucose, fructose, galactose), disaccharides (sucrose, lactose, maltose), and trisaccharides (raffinose). Many monosaccharides and disaccharides fairly commonly found in nature bear names reflecting the source from which they were first isolated. For example, glucose is also known as grape sugar, lactose as milk sugar, and maltose as malt sugar. In everyday usage, the name is often used to refer specifically to sucrose (table sugar, cane sugar, beet sugar).
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
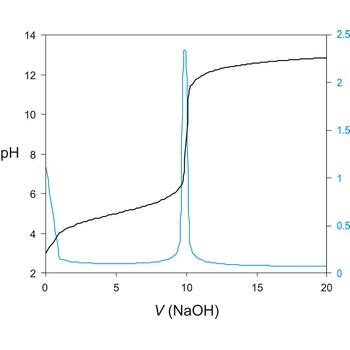
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
toxin → toksin
Toxins are effective and specific poisons produced by living organisms. They usually consist of an amino acid chain which can vary in molecular weight between a couple of hundred (peptides) and one hundred thousand (proteins). They may also be low-molecular organic compounds. Toxins are produced by numerous organisms, e.g., bacteria, fungi, algae and plants. Many of them are extremely poisonous, with a toxicity that is several orders of magnitude greater than the nerve agents. Botulinum toxin, produced by the bacteria Clostridium botulinum, is the most poisonous substance known.
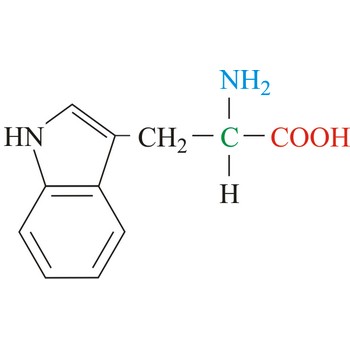
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
Citing this page:
Generalic, Eni. "Mil-spec." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table