period → perioda
Periods are horizontal rows in the periodic table, each period begin with an alkali metal (one electron in the outermost principal quantum level) and ending with a noble gas (each having eight electrons in the outermost principal quantum level, except for helium, which is limited to two).
pickling → dekapiranje
Pickling is a process to chemically remove scale or oxide from steel to obtain a clean surface. When applied to bars or coils prior to bright drawing, the steel is immersed in a bath of dilute sulphuric acid (w(H2SO4) = 10 %) heated to a temperature of around 80 °C. An inhibitor is added to prevent attack and pitting of the cleaned metal. After pickling, a washing process takes place followed by immersion in a lime-water bath to neutralise any remaining acid.
platinum → platina
Platinum was discovered by Antonio de Ulloa (South America) in 1735. The origin of the name comes from the Spanish word platina meaning silver. It is rare, very heavy, soft, silvery-white metal. Resists oxygen and water. Platinum is produced from deposits of native, or elemental. Used in jewellery, to make crucible and special containers and as a catalyst. Used with cobalt to produce very strong magnets. Also to make standard weights and measures. Resists corrosion and acid attacks except aqua regia.
plutonium → plutonij
Plutonium was discovered by Glenn T. Seaborg, Edwin M. McMillan, J. W. Kennedy and A. C. Wahl (USA) in 1940. Named after the planet Pluto. It is silvery-white, extremely radioactive artificially produced metal. Reacts with oxygen and acids; resists alkalis. Attacked by steam. Highly toxic. Plutonium is found rarely in some uranium ores. Made by bombarding uranium with neutrons. Used in bombs and reactors. Small quantities are used in thermo-electric generators.
poison → otrov
Poisons are substance, which upon contact or being introduced into an organism, impair or prevent normal metabolic processes from taking place, thus altering the normal functioning of organs or tissues.
Poisons are molecules or material that tends to collect on a catalyst surface, blocking access to active sites or destroying their activities.
Poisons are substance that can reduce a nuclear reaction by absorbing neutrons, thereby preventing more fission. If enough poisons are present in a reactor core, the chain reaction will die out.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
polydentant ligand → polidentantni liganad
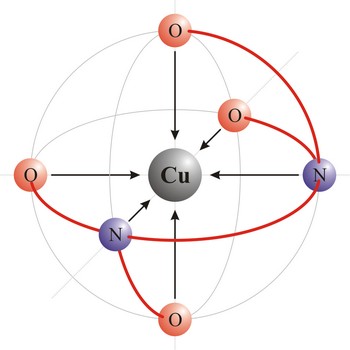
Polydentant ligands contain more co-ordination points (can give more electron pairs) and they form complex ringlike structures (celate complexes) by replacing two or more monodentant ligands. That kind of ligand is EDTA which has 6 co-ordinational points and with metals it creates complexes, always in 1:1 ratio.
polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
Citing this page:
Generalic, Eni. "Metar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table