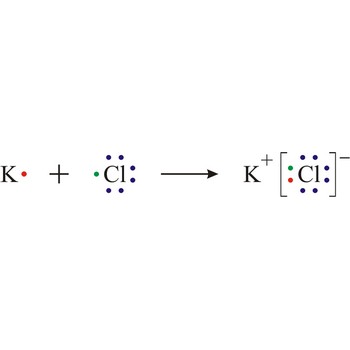lattice → kristalna rešetka
Crystal lattice is a three-dimensional array of points that embodies the pattern of repetition in a crystalline solid. Don’t mix up atoms with lattice points: lattice points are infinitesimal points in space - atoms are physical objects.
liquid crystal → tekući kristal
Liquid crystals or crystalline liquids are a physical state between crystals and melts. The liquid crystalline phase - the so-called mesophase - is formed at the melting point. The most important (usable) mesophases are nematic, cholesteric and smectic phase, having different molecular orientations.
fluorine → fluor
Fluorine was discovered by Henri Moissan (France) in 1886. The origin of the name comes from the Latin word fluere meaning to flow. It is pale yellow to greenish gas, with an irritating pungent odour. Extremely reactive, flammable gas. Reacts violently with many materials. Toxic by inhalation or ingestion. Does not occur uncombined in nature. Fluorine is found in the minerals fluorite (CaF2) and cryolite (Na3AlF6). Electrolysis of hydrofluoric acid (HF) or potassium acid fluoride (KHF2) is the only practical method of commercial production. Used in refrigerants and other fluorocarbons. Also in toothpaste as sodium fluoride (NaF).
free radical → slobodni radikal
Free radical is a molecular fragment having one or more unpaired electrons, usually short-lived and highly reactive. They can be produced by photolysis or pyrolysis in which a bond is broken without forming ions. In formulas, a free radical is conventionally indicated by a dot (·CH3, ·SnH3, ·Cl). Free radicals are known to be formed by ionising radiation and thus play a part in deleterious degradation effects that occur in irradiated tissue. They also act as initiators or intermediates in oxidation, combustion, photolysis, and polymerisation.
kelvin → kelvin
Kelvin (K) is the SI base unit of thermodynamic temperature.
The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The unit was named after the British scientist Sir. W. Thompson, Lord Kelvin (1824-1907).refractory metal → vatrostalni metal
Refractory metal is a metal having an extremely high melting point, for example tungsten, molybdenum, niobium, tantalum, and rhenium
terminal → terminalni
Terminal in chemistry means: the end of a polymer molecule and a point at which electron connections can easily be made or broken.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
Citing this page:
Generalic, Eni. "Materijalna točka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table