activity coefficient → koeficijent aktiviteta
Activity coefficient (γ or f) is a fractional number which, when multiplied by the molar concentration of a substance in solution, yields the chemical activity. This term gives an idea of how much interaction exists between molecules at higher concentration.
In solutions of very low ionic strength, when m is less than 0.01, the Debye-Hückel limiting law can be used to calculate approximate activity coefficients
where γi = activity coefficient of the species i, zi = charge on the species i and μ = ionic strength of the solution.
average rate of reaction → prosječna brzina reakcije
Average rate of reaction is calculated in a way that a total change of reactants and products concentration is divided with time which is needed for reaction to end.
battery acid → baterijska kiselina
Battery acid is a solution of approximately 6 mol L-1 sulphuric acid used in the lead storage battery
Beer’s law → Beerov zakon
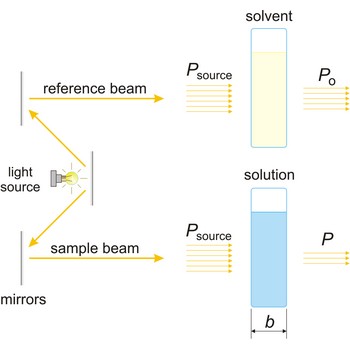
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
beryllium → berilij
Beryllium was discovered by Friedrich Wöhler (Germany) and independently by A. B. Bussy (France) in 1828. The origin of the name comes from the Greek word beryllos meaning mineral beryl; also called glucinium from the Greek word glykys meaning sweet. It is steel-grey metal. It resists attack by concentrated nitric acid, has excellent thermal conductivity and is nonmagnetic. At ordinary temperatures, it resists oxidation in air. Beryllium and its salts are toxic and should be handled with the greatest of care. Beryllium is found mostly in minerals like beryl [AlBe3(Si6O18)] and chrysoberyl (Al2BeO4). Pure beryllium is obtained by chemically reducing beryl mineral. Also by electrolysis of beryllium chloride. Its ability to absorb large amounts of heat makes it useful in spacecraft, missiles, aircraft, etc. Emeralds are beryl crystals with chromium traces giving them their green colour.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
coagulation → koaguliranje
Coagulation is a process of colloid particles merging into bigger ones. By removing the charge form a colloid ion, by increasing the temperature or by increasing electrolyte concentration, colloid particles will gather into bigger groups precipitate will emerge. Precipitate that is formed in this way (coagulate) is amorphic and considerably polluted with adsorbed pollutants.
colorimetry → kolorimetrija
Colorimetry is a quantitative chemical analysis by colour using a colorimeter.
dilute solution → razrijeđena otopina
Dilute solution contains a relatively low concentration of solute.
Citing this page:
Generalic, Eni. "Masena koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table