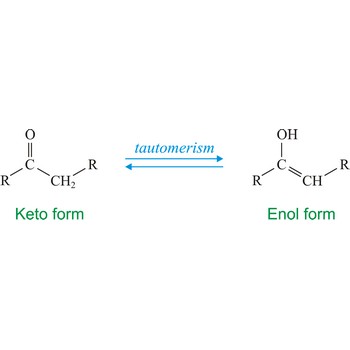
tautomerism → tautomerija
Tautomerism refers to equilibrium between two different structures of the same compound. Usually the tautomers differ in the point of attachment of a hydrogen atom. One of the most common examples of a tautomeric system is the equilibrium between a ketone (keto) and aldehyde (enol).
tear gas → suzavac
Tear gases is the common name for substances which, in low concentrations, cause pain in the eyes, flow of tears and difficulty in keeping the eyes open. Tear gases are used mainly in military exercises and in riot control, etc., but have also been used as a method of warfare. Irritating gases have been used in war since ancient times but it was not until after the Second World War that a more systematic search for effective substances was started. Among a long series of substances, three have become of greater importance than the others. These substances are chloroacetophenone (codename CN), orto-chlorobenzylidene-malononitrile (CS) and dibenz(b,f)-1,4-oxazepine (CR).
technetium → tehnecij
Technetium was discovered by Carlo Perrier and Emilio Segre (Italy) in 1937. The origin of the name comes from the Greek word technikos meaning artificial. It is silvery-grey metal. Resists oxidation but tarnishes in moist air and burns in high oxygen environment. First synthetically produced element. Radioactive. Technetium is made first by bombarding molybdenum with deuterons (heavy hydrogen) in a cyclotron. Added to iron in quantities as low as 55 part-per-million transforms the iron into a corrosion-resistant alloy.
teflon → teflon
Teflon is a DuPont Company trademark for polytetrafluoroethylene (PTFE). It was discovered in 1938 by R. J. Plunkett. Teflon has the lowest coefficient of friction of any solid material known to man. It is also used as a non-stick coating for pans and other cookware. Teflon is very unreactive, and so is often used in containers and pipework for reactive chemicals. Its melting point is 327 °C.
tellurium → telurij
Tellurium was discovered by Franz Joseph Muller von Reichstein (Romania) in 1782. The origin of the name comes from the Latin word tellus meaning earth. It is silvery-white, brittle semi-metal. Unreactive with water or HCl; dissolves in HNO3; burns in air or oxygen. Tellurium is obtained as a by-product of copper and lead refining. Used to improve the machining quality of copper and stainless steel products and to colour glass and ceramics. Also in thermoelectric devices. Some is used in the rubber industry and it is a basic ingredient in manufacturing blasting caps.
temporary hardness → prolazna tvrdoća
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. It can be removed by water reboiling, whereby white solid emerges calcium carbonate that is limescale.
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
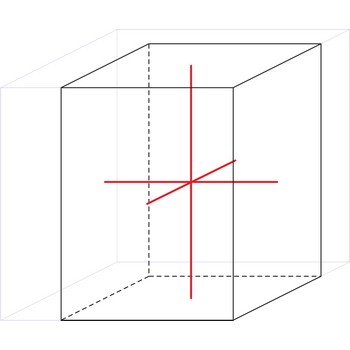
tetragonal crystal system → tetragonski kristalni sustav
Minerals of the tetragonal crystal system are referred to three mutually perpendicular axes. The two horizontal axes are of equal length, while the vertical axis is of different length and may be either shorter or longer than the other two.
a = b ≠ c
α = β = γ = 90°
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
thallium → talij
Thallium was discovered by Sir William Crookes (England) in 1861. The origin of the name comes from the Greek word thallos meaning green twig or green shoot. It is soft grey metal that looks like lead. Tarnishes in moist air. Reacts in heated moist air and in acids. Compounds highly toxic by inhalation or ingestion. Cumulative effects. Thallium is found in iron pyrites. Also in crookesite, hutchinsonite and lorandite. Most is recovered from the by-products of lead and zinc refining. Its compounds are used in rat and ant poisons. Also for detecting infrared radiation.
Citing this page:
Generalic, Eni. "Ledište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table